
ASCO 2025 – 3SBio reveals what Pfizer got for its $1.25bn
Early data on SSGJ-707 in PD-L1-positive lung cancer spur comparisons against ivonescimab.
Early data on SSGJ-707 in PD-L1-positive lung cancer spur comparisons against ivonescimab.

Judging by the activity around Saturday's ASCO poster covering 3SBio's anti-PD-1 x VEGF MAb SSGJ-707, licensed this month by Pfizer, there is at least a moderate amount of interest in finding out what the US big pharma group got for the $1.25bn it paid up front.
The answer seems to be: a molecule with impressive activity in first-line lung cancer, spread fairly evenly between PD-L1 1-49% expressers and PD-L1-high disease, but one that also brings its share of toxicity. In particular, one adverse event-related discontinuation and one death among 12 patients in the 20mg/kg group has resulted in 10mg/kg being the dose taken forward.
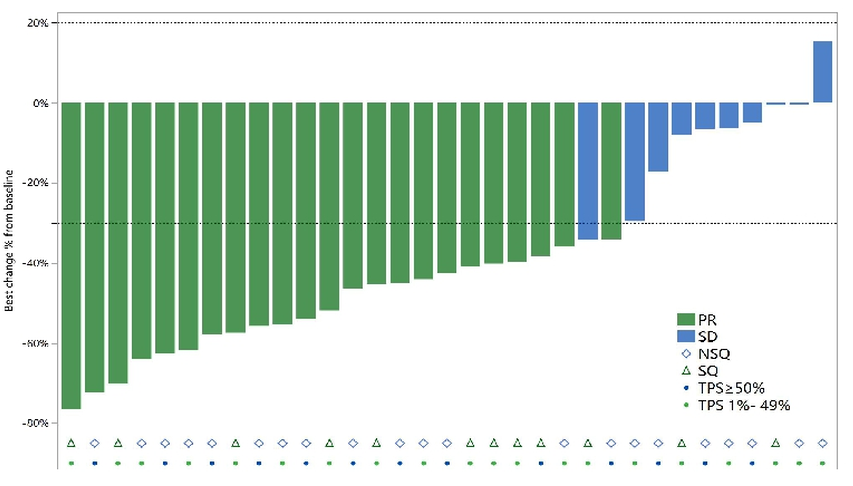
In this Chinese study, the headline confirmed overall response rate number of 41% among 83 efficacy-evaluable patients thus probably matters less than the number specifically among the 34 subjects given 10mg/kg: 65%.
This reveals a clear lack of dose response, with ORRs in 11 20mg/kg patients and four 30mg/kg subjects being just 36% and 25% respectively. However, if this is an artefact of low patient numbers, 65% seems an impressive ORR number; the highest-profile PD-1 x VEGF contender, Akeso/Summit's ivonescimab, scored an ORR of 50% in the Chinese phase 3 Harmoni-2 study in a similar first-line PD-L1-positive population.
This is also good news given that one of the few numbers investors have had to go on so far has been a 71% ORR for SSGJ-707 10mg/kg, dribbled out in 3SBio’s 2024 earnings presentation. The ASCO poster's waterfalls for 10mg/kg, relating to unconfirmed as well as confirmed responses, show response rates of 65% among 1-49% PD-L1 expressers, and 77% among ≥50% expressers.
Activity of SSGJ-707 at 10mg/kg
For its part, the 2024 World Lung presentation of Akeso's Harmoni-2 trial of ivonescimab didn't split responses by PD-L1 status. However, the hazard ratio for PFS with ivonescimab versus Keytruda was slightly more impressive in PD-L1 ≥50% (0.48) than in 1-49% (0.54) patients.
Ivonescimab is a notable absentee from this year's ASCO. However, Summit did attempt to gatecrash the conference by releasing topline data from the Harmoni trial by press release on ASCO's first day – an effort that investors rewarded with a 30% share price fall.
Safety
So what about the safety of SSGJ-707? The poster reveals a 33% rate of grade 3 or higher treatment-related adverse events across all patients, 8% rate of TRAE-related discontinuations, and one TRAE leading to death (in the 20mg/kg group). Asked by ApexOnco the team responsible for the poster wasn't able to confirm the reasons for the discontinuations and death.
However, the most common TRAEs with the 10mg/kg dose specifically were spelled out as AST increase, ALT increase, proteinuria, hypothyroidism, hypertriglyceridaemia, hypercholesterolaemia, hypocalcaemia, haemoptysis and hypertension.
These are perhaps not unexpected. Harmoni-2 revealed a 29% rate of grade 3 or higher TRAEs among 197 ivonescimab patients, a 21% rate of serious events, three leading to discontinuation and one leading to death.
Those interested in SSGJ-707's chemical structure will note the claim made in the ASCO poster that in the presence of VEGF the molecule's affinity for PD-1 is "100-fold increased compared to (its) parent PD-1 antibody".
With a Chinese phase 3 trial of SSGJ-707, in first-line PD-L1-positive NSCLC, listed on clinicaltrials.gov, and Pfizer yet to reveal its US plans for the molecule, the debate over the reproducibility of Chinese data in an ex-China population – for ivonescimab and SSGJ-707 alike – will rumble on.
6743













