
ASCO 2025 – Astra "looked at" patritumab but passed
As patritumab-dxd's problems mount, Astra's decision to say no to this project looks smart.
As patritumab-dxd's problems mount, Astra's decision to say no to this project looks smart.

Before Merck & Co blew $1.5bn on Daiichi Sankyo's patritumab deruxtecan in a multi-project 2023 deal AstraZeneca also considered licensing this anti-HER3 ADC, it has emerged. However, Astra decided that a deal didn't make sense – a stance vindicated by the multiple problems patri-dxd now faces, including a withdrawn FDA filing and disappointing phase 3 data revealed on Sunday at ASCO.
Astra itself had an earlier tie-up with Daiichi, covering Enhertu and Datroway, and "we looked at patritumab-dxd, but decided not to move forward with it", Astra's chief executive, Pascal Soriot, told ApexOnco in response to a question at a pre-ASCO media briefing. Soriot's reluctance was partly down to patri-dxd's toxicity, something the ASCO data now show to be a live issue.
Results of patri-dxd's potentially confirmatory phase 3 Herthena-Lung02 study, in second-line EGFR-mutated lung cancer, caused the project's US application for accelerated approval to be withdrawn last week. The full data presented for the first time at ASCO by Chinese University of Hong Kong's Dr Tony Mok showed why.
The OS curves actually suggested a better outcome for the platinum chemo comparator before the median time point, before crossing over. For the record, median OS was 16.0 months for patri-dxd versus 15.9 months for platinum chemo, and the hazard ratio was a clearly non-significant 0.98. One positive is that patri-dxd wasn't associated with a greater risk of death than chemo across the whole study.
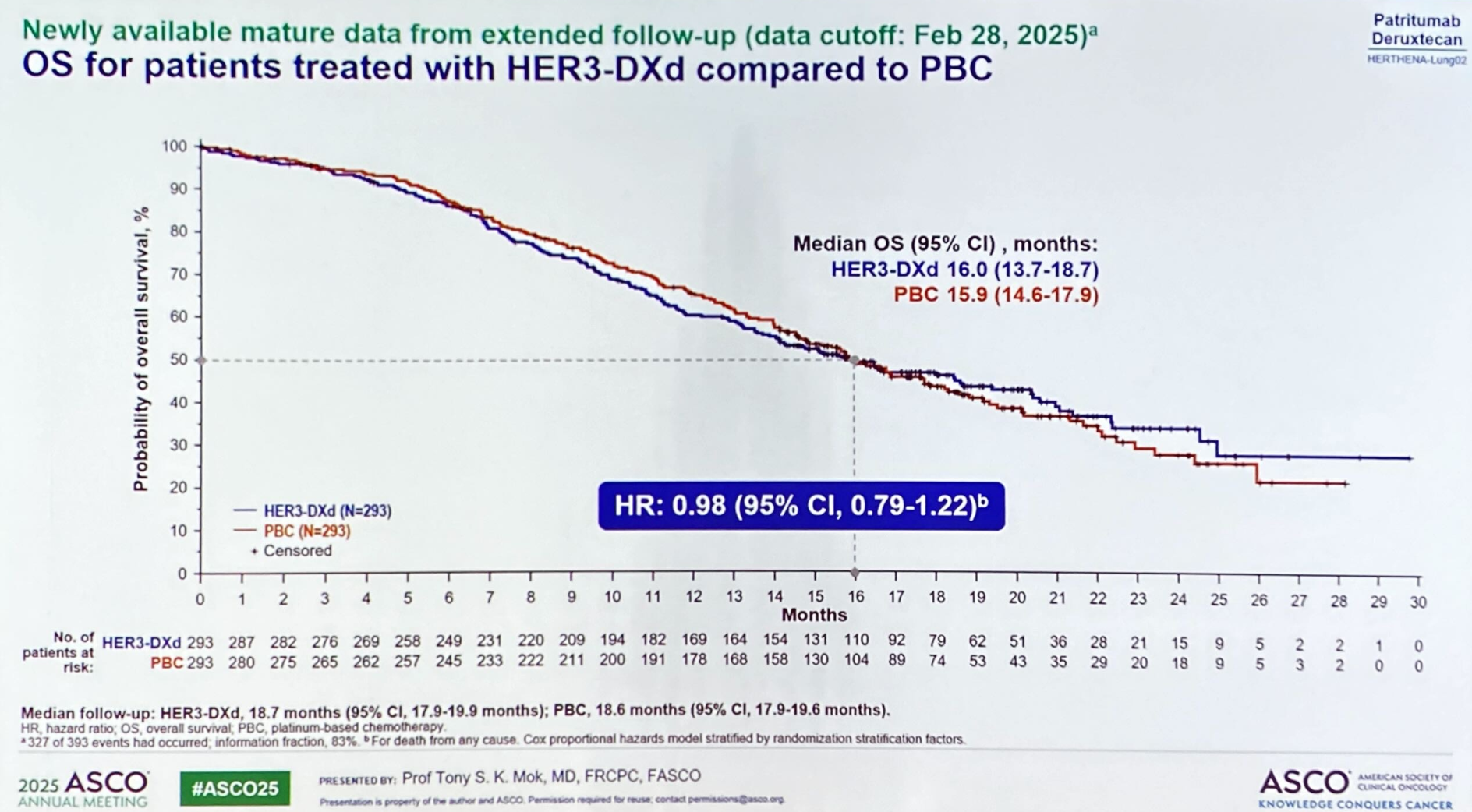
The OS data reached maturity with 327 of 393 patient deaths recorded, which triggered a third interim analysis on 28 February 2025, Mok disclosed. That was after Herthena-Lung02 hit the required number of progression events on 31 May 2024, leading to it being toplined as positive for PFS, its primary endpoint, last September.
Mok also revealed the resulting PFS curves, which showed near superimposability until the median, where a benefit of just 0.4 months was seen. It was only later that these curves separated, giving a 0.77 hazard ratio (p=0.011). This type of survival curve often suggests the presence of biomarkers, but the best Mok could say was that evaluation of predictive biomarkers was ongoing.
Not clinically significant
While Mok stated factually that the PFS benefit was statistically significant, the ASCO discussant, Tata Memorial Hospital's Dr Vanita Noronha was harsher, calling the PFS benefit minimal, and saying it was "perhaps not clinically significant".
She also slammed patri-dxd's "substantial" toxicity, which she said would have rendered the drug inappropriate for frail patients if it had been approved. Tox amounted to a 58% rate of grade 3 or higher treatment-related adverse events, leading to dose reduction in 32% of patients, and treatment discontinuation in 11%.
Mok shed more light on grade 5 events referred to in the ASCO abstract, saying four patients died as a result of patri-dxd, two from interstitial lung disease (ILD), one from pneumonia and one from septic shock. In total there were 14 ILD cases, including one at grade 3. In the chemo cohort there was one death, from cerebral haemorrhage.
While Merck now has to decide how to proceed with patri-dxd, Astra seems to have dodged a bullet. Soriot said there was a variety of reasons why he decided against licensing this ADC from Daiichi, including the fact patri-dxd didn't combine well with Tagrisso. But on one point he was clear, stating: "There were too many toxicities."
4317













