ASCO 2025 – Astra's Matterhorn could break new ground
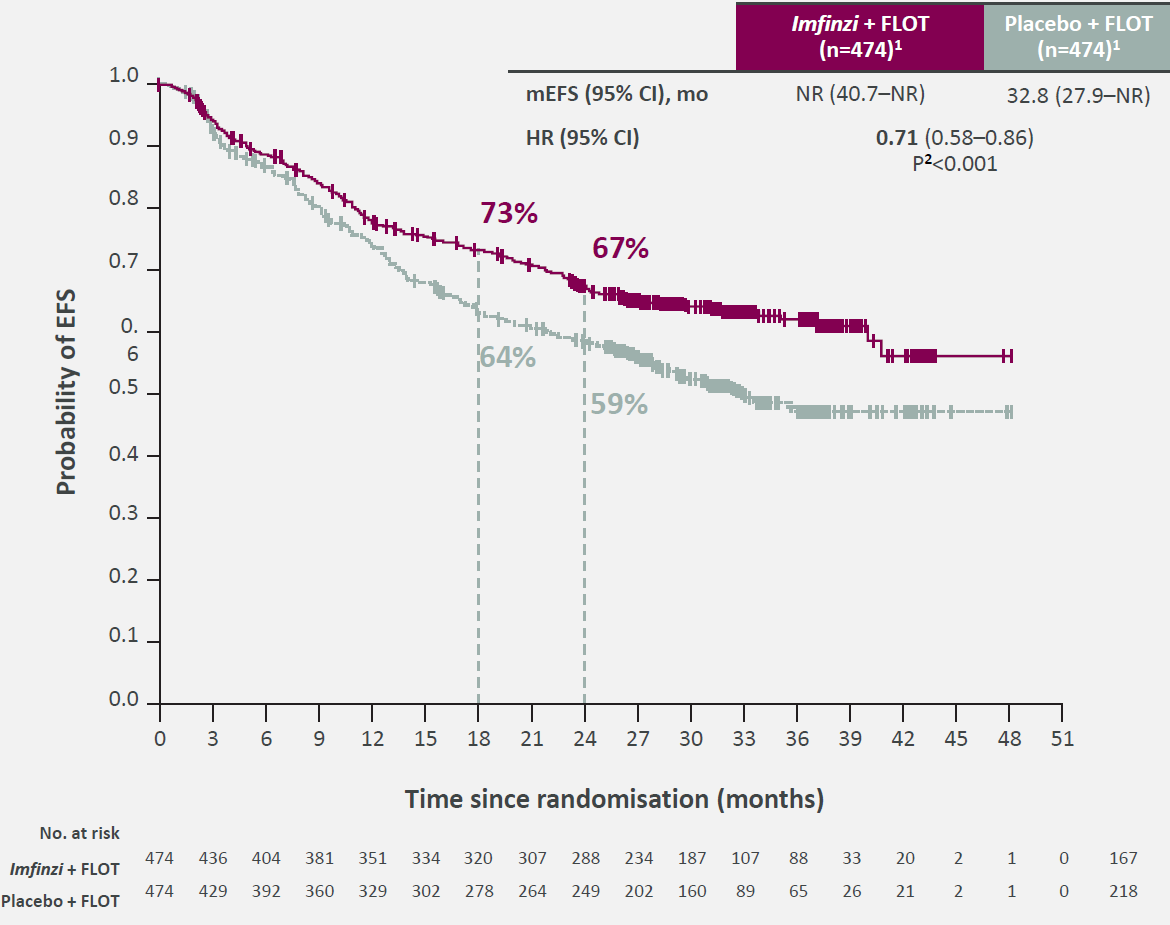
AstraZeneca hopes that the event-free survival result from the Matterhorn trial, just unveiled at ASCO's plenary session, will enable Imfinzi to become the first immuno-oncology drug approved for the perioperative treatment of stomach cancer. Precise filing plans haven't been revealed, but if Astra succeeds it will have beaten Merck & Co, whose analogous Keynote-585 study of Keytruda failed on a statistical quirk. Importantly, the full EFS data from Matterhorn appear to be better than those from Keynote-585; though the Matterhorn result is relatively immature, with no median yet hit for Imfinzi plus chemo, the hazard ratio for EFS has come in at 0.71, against Keynote-585's 0.81. Moreover, control did better in the Astra study, with median EFS of 32.8 months as opposed to 25.3 months in Keynote-585. One unknown is the stance of the FDA, which has criticised perioperative studies that, like Matterhorn, don't split out the benefit of the adjuvant and neoadjuvant stages. However, speaking at a pre-ASCO media briefing Astra’s head of oncology R&D, Susan Galbraith, said that "for ongoing trials that was unlikely to be an issue. In gastric cancer already the chemotherapy is given in a perioperative design, and that was recapitulated in Matterhorn."
Matterhorn event-free survival curves
2557













