
ASH 2025 – J&J’s multiple myeloma juggling act
Tecvayli plus Darzalex could challenge Carvykti in the push for a functional cure.
Tecvayli plus Darzalex could challenge Carvykti in the push for a functional cure.

Johnson & Johnson had already impressed with its Tecvayli-Darzalex combo in second to fourth-line multiple myeloma, and the ASH late-breaking presentation on Tuesday from the Majestec-3 trial showed why the company is talking about a functional cure.
The just-revealed progression-free survival curves separate early and almost plateau with the combination, leading investigators to conclude in a simultaneous NEJM publication: “We may be entering a new era of resetting survival expectations in a cancer that has been historically described as incurable.”
The authors also included J&J and Legend’s BCMA-targeting Car-T therapy Carvykti in this new era, suggesting that J&J might be about to cannibalise its own product.
As already revealed in the ASH abstract, Tecvayli (a BCMA-targeting T-cell engager) plus Darzalex (an anti-CD38 MAb) reduced the risk of progression or death by 83% versus Darzalex plus dexamethasone and either Pomalyst or Velcade.
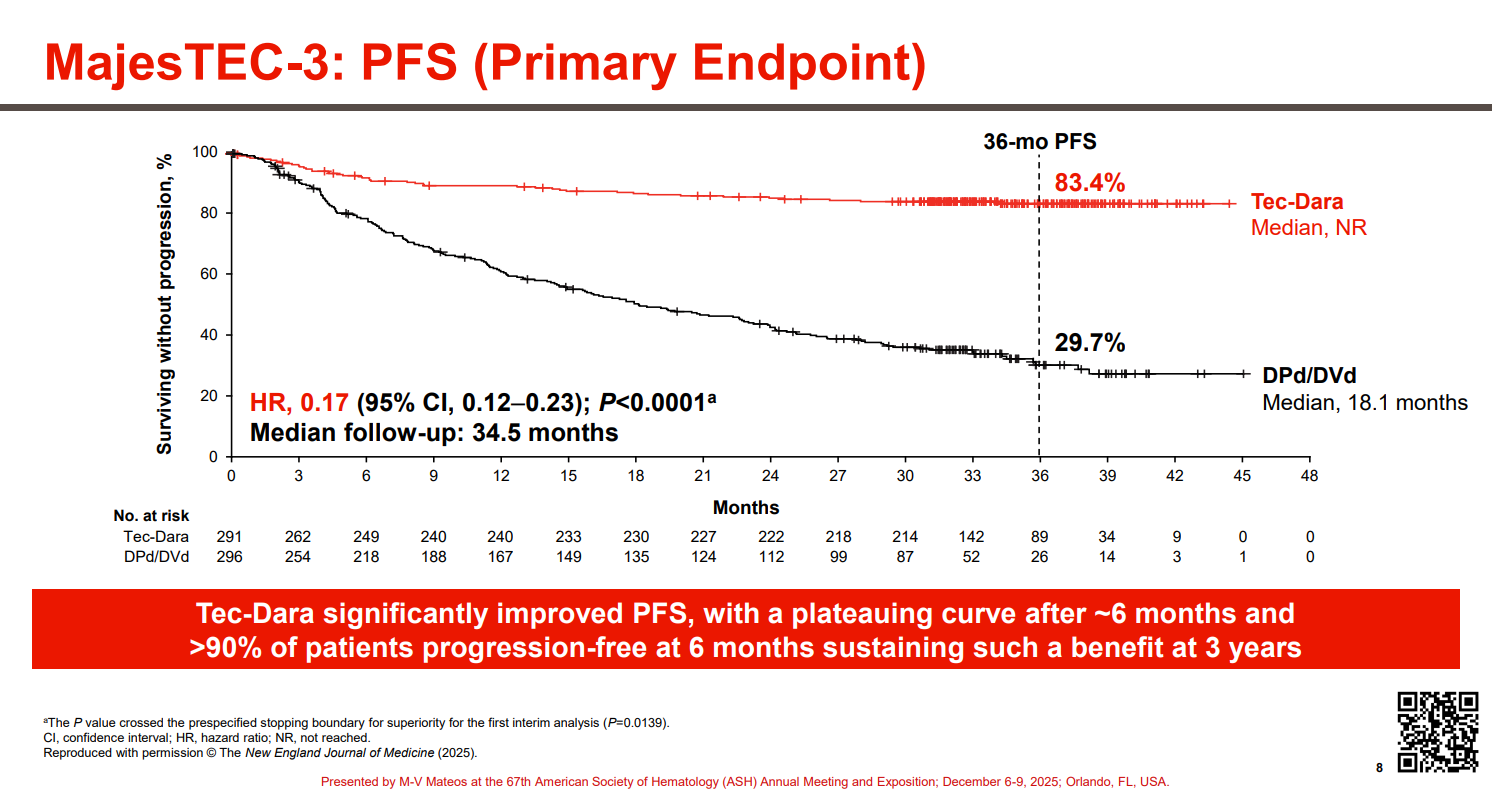
Source: Dr Maria Victoria Mateos & ASH.
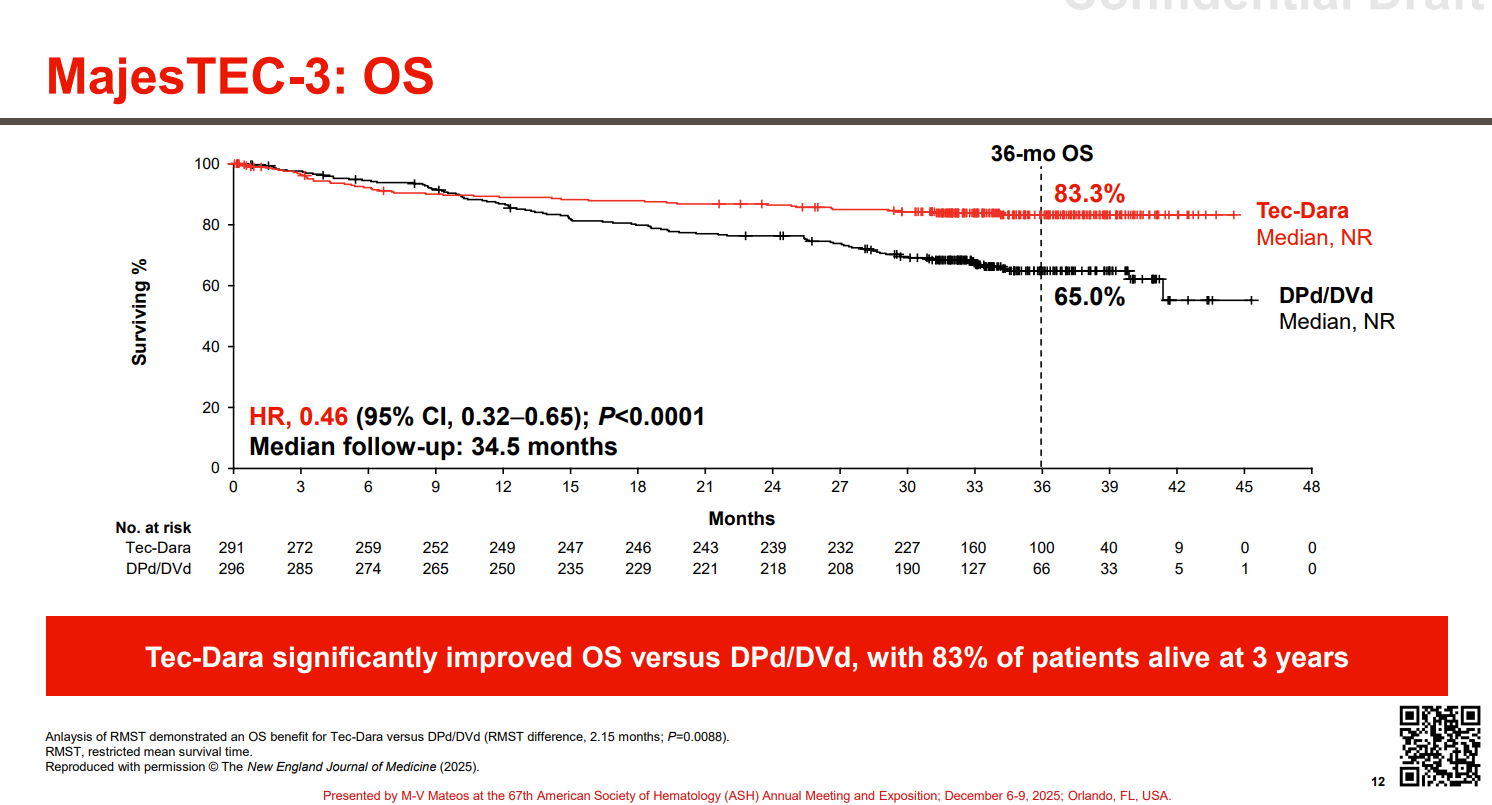
However, one fly in the ointment could be the crossing over of the OS curves – which the researchers put down to early deaths from infection in the Tecvayli plus Darzalex group. Fatal infections occurred in 13 patients (5%) in this cohort, versus 4 (1%) in the control arm.
Still, 12 of the 13 infection-related deaths with Tecvayli plus Darzalex occurred before the trial’s protocol was amended to reinforce immune globulin treatment and antimicrobial prophylaxis, to help prevent infections. Only one infection-related death occurred after this amendment.
And, despite this crossover, OS was statistically significantly improved with Tecvayli plus Darzalex, with a hazard ratio of 0.46.
The authors conclude that adverse events “can be ameliorated with the use of established protocols”, something that will have to be borne out in the real world.
Tecvayli currently has accelerated approval as monotherapy in fifth-line multiple myeloma. J&J has now filed Tecvayli-Darzalex with the FDA; the combo has breakthrough designation and is being reviewed under the real-time oncology review programme.
If the combo is approved in earlier lines patients could be faced with a choice between Tecvayli-Darazelx and Carvykti in second-line multiple myeloma; the Car-T was approved for this use in April 2024.
“Both offer the same kind of opportunity of working towards cure,” J&J’s vice-president of global medical affairs, Mark Wildgust, told ApexOnco in a pre-ASH briefing. He noted that Carvykti offers a one-and-done therapy, while Tecvayli-Darzalex would be more suited for patients who don’t want to travel, or can’t get access to the Car-T centre, as it would be delivered in a community-based setting.
He added: “There are 128 places in the US that you can get Carvykti, but there are more than 5,000 centres that use Darzalex.” Carvykti sold $963m in 2024, while Tecvayli brought in $549m.
3950













