
Bristol challenges Adcetris on its own turf
Some time ago Opdivo beat Adcetris in an NCI trial, and now Bristol files.
Some time ago Opdivo beat Adcetris in an NCI trial, and now Bristol files.

Classical Hodgkin’s lymphoma remains a niche indication for anti-PD-(L)1 drugs, and one where Pfizer/Takeda’s Adcetris is a key front-line treatment, but if Bristol Myers Squibb has anything to do with it this could soon change.
Bristol surprised the markets on Thursday by revealing the US FDA filing of an Opdivo chemo combo for first-line classical Hodgkin’s. The reason this was surprising was that the company doesn’t ever appear to have sponsored a pivotal trial of its drug in this setting, and instead is backing its submission with a lower-profile academic study called SWOG S1826.
Perhaps thanks to not being an industry-sponsored effort, SWOG S1826 had a bold design, pitching Opdivo plus doxorubicin, vinblastine and dacarbazine head to head against Adcetris with the same chemo regimen. Adcetris plus doxorubicin, vinblastine and dacarbazine is a first-line standard of care, having been US approved in 2018 on the back of the Echelon-1 trial, where it beat bleomycin/doxorubicin/vinblastine/dacarbazine on PFS.
Three-year PFS
SWOG S1826 thus aims to put the cat among the pigeons, but interestingly this trial, sponsored by the US NCI, had already read out positively for PFS at ASCO two years ago. Bristol appears to have wanted to wait for more mature data to confirm Opdivo’s benefit, and fortunately this came in an ASH presentation a week ago.
That presentation related to a three-year follow-up from SWOG S1826. With 487 efficacy-evaluable patients assigned to Opdivo plus chemo, and 483 to Adcetris/chemo, the PFS curves are continuing to show separation, amounting to a nine-point benefit favouring Opdivo at the three-year landmark, and a 52% reduction in risk of progression or death (p<0.0001).
PFS curves from SWOG S1826
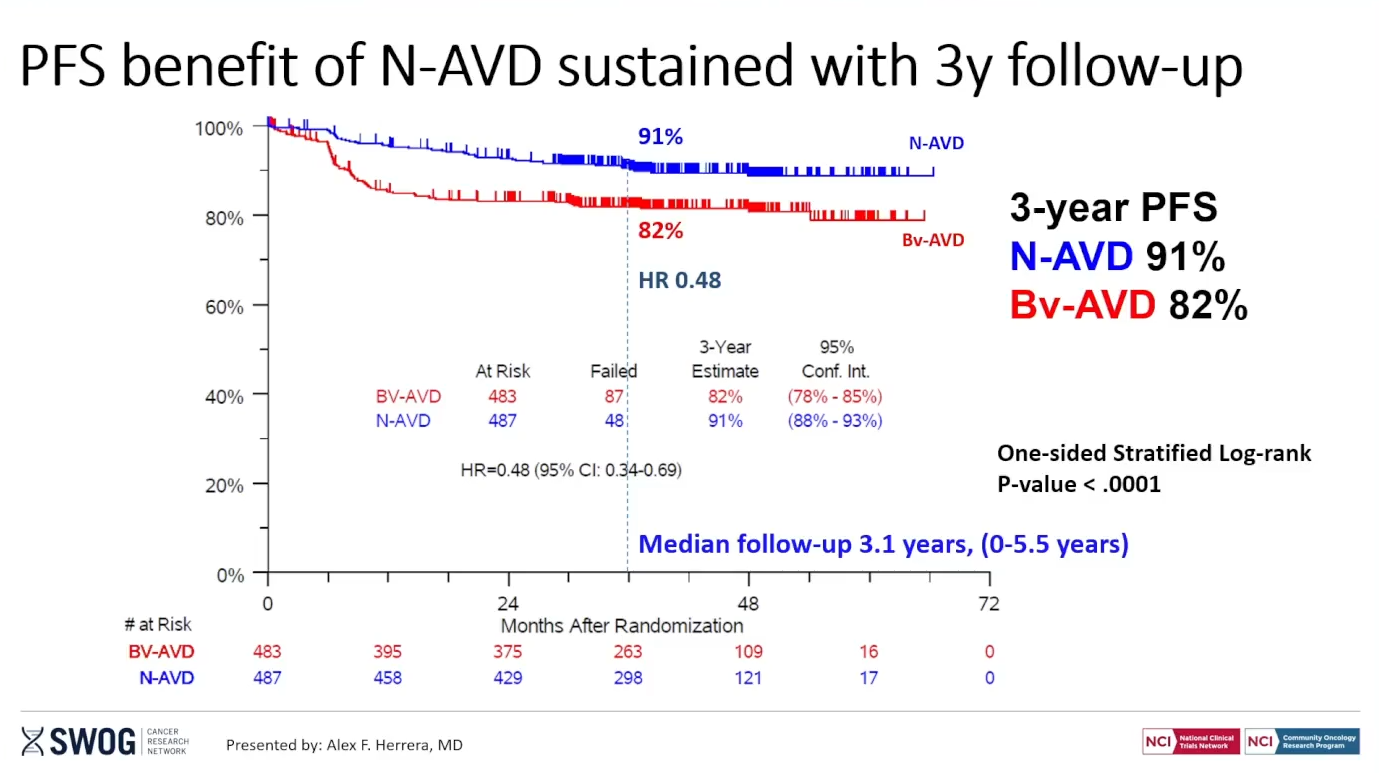
The trial's active control is in line with Echelon-1, whose Adcetris/chemo active cohort yielded a three-year PFS rate of 83%. Adcetris’s first-line label cites a 23% reduction in risk of progression or death for the Pfizer/Takeda drug’s chemo combo versus the bleomycin-containing regimen.
The FDA has set an 8 April 2026 PDUFA date by which it aims to issue a verdict on Bristol’s filing.
So far the only ingress into classical Hodgkin’s lymphoma by PD-(L)1 drugs in the US has come from Opdivo and Keytruda monotherapy; the former is approved third line, while the latter was approved fourth line, before in 2020 scoring a label in stem cell transplant-relapsed patients. Five drugs hold Chinese third-line approvals, including Innovent’s Tyvyt and BeOne’s Baizean.
Presenting the SWOG S1826 data at ASH, City of Hope’s Dr Alex Herrera said Opdivo’s PFS benefit held up across age groups and disease severity scores, and was also backed by an advantage versus Adcetris on event-free survival, which captures more events than PFS.
Overall survival, a metric Adcetris’s prescribing information is already able to boast of in Echelon-1 (0.59 hazard ratio), is still very immature in SWOG S1826, with only eight deaths so far on Opdivo, and 15 on Adcetris.
1138













