
EHA 2025 – ELVN-001 enlivens investors
The Enliven molecule looks competitive against Scemblix, triggering a $200m raise.
The Enliven molecule looks competitive against Scemblix, triggering a $200m raise.

With Enliven Therapeutics's BCR-ABL inhibitor ELVN-001 showing meaningful activity in chronic myelogenous leukaemia patients who progress on Novartis's Scemblix the scene is set for Enliven investors' bull case that ELVN-001 becomes a cornerstone of CML treatment.
Whether this happens depends on far more data, as well as regulatory execution, but for now Enliven has done enough to please the markets: phase 1 ELVN-001 data, presented at the European Hematology Association congress over the weekend but revealed to investors on Friday, drove Enliven stock up 11% and allowed the group to close a $200m equity raise.
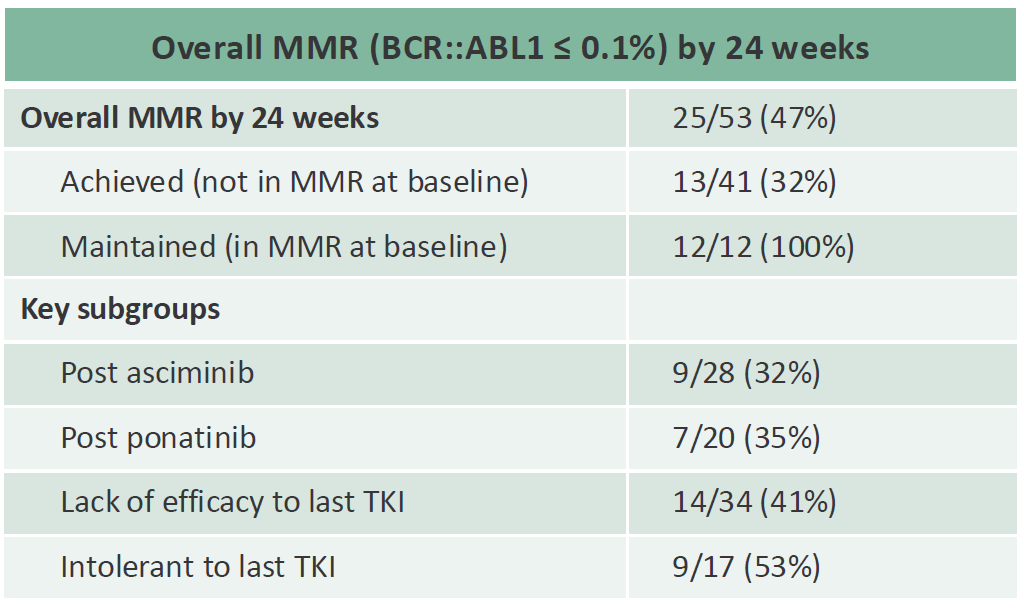
Enliven's investment case is now entirely dependent on ELVN-001, and the EHA abstract showed the first hints of promise, detailing a 24-week major molecular response (MMR) rate of 44% among 36 late-line CML patients at a 21 January cutoff. Full data, with a 28 April cutoff, showed an MMR rate of 47% in 53 patients (including 12 who were in MMR at baseline).
Enliven also presented a cross-trial comparison, showing that its 47% MMR rate was numerically better than the 37% Scemblix had scored in a phase 1 trial in a similar CML setting published in 2019, with the added bonus of ELVN-001's study having been run in what looks like a more heavily pretreated population. Scemblix is a key comparator because of its ability to overcome resistance mechanisms to early-generation BCR-ABL inhibitors.
However, possibly Enliven's most important data point was that 28 patients in the ELVN-001 study were post-Scemblix, and 32% of these were in MMR at week 24. All the abstract had revealed was that the MMR rate in patients relapsed on Scemblix or Takeda's Iclusig was 36%, without splitting out Scemblix failures.
This is a key metric because of Scemblix's recent approval for first-line CML. The Novartis drug looks likely now to become a front-line standard, and if this happens then a molecule that, like ELVN-001, might overcome resistance to it could accordingly move forward in the treatment hierarchy.
ELVN-001 24-week efficacy data summary
As for safety, the EHA data revealed a 20% discontinuation rate in the ELVN-001 trial (18 of 90 patients), slightly higher than the 17% 24-week discontinuation rate seen in Scemblix's Ascembl study. Grade 3 arterial occlusive events were reported in two patients, but were deemed unrelated to ELVN-001, with both patients remaining on trial.
This is good news with an early dataset, but of course Enliven must now show similar promise in a phase 3 trial. The company has outlined plans broadly to follow Scemblix's development plan, firstly by running a study in third or second-line CML, comprising a head-to-head comparison against Bosulif, or physician's choice including Gleevec, respectively.
This pivotal study is planned to start next year. There is then the possibility of beginning a front-line trial, replicating Scemblix's ASC4First study, though this is still on a back burner and depends on evolving data continuing "to be supportive", Enliven says.
For now the $200m raise, comprising a sale of new shares and warrants, gives Enliven a cash runway into the first half of 2029, a timeline that should get the company past its first phase 3 readout.
1363













