
ESMO 2025 – Lilly strengthens Verzenio’s case
The company reveals the first overall survival data in adjuvant ER-positive breast cancer.
The company reveals the first overall survival data in adjuvant ER-positive breast cancer.

Two months after revealing that the MonarchE trial of Verzenio had hit its overall survival goal, Lilly is filling in the blanks.
At ESMO on Friday the company revealed the full seven-year results from MonarchE, offering the first detailed look at the CDK4/6 inhibitor’s overall survival advantage in ER-positive, HER2-negative high-risk early breast cancer.
Adjuvant breast
Verzenio has been approved for the adjuvant treatment of adults with ER-positive, node-positive, early breast cancer at high risk of recurrence for several years now, based on iDFS data from the MonarchE study.
But its main CDK4/6 rival, Novartis’s Kisqali gained a broader US label last September, including in node-negative patients, based on its Natalee trial.
With roughly 40% of newly diagnosed breast cancer patients potentially eligible to be treated with a CDK4/6 inhibitor, Lilly’s ability to show a statistically significant overall survival benefit could therefore give Verzenio a slight advantage over Kisqali; Natalee has yet to yield overall survival data.
The MonarchE results also bring some redemption for Lilly’s CDK4/6 inhibitor, coming after the Monarch-3 trial in first-line advanced breast cancer failed to show a statistically significant improvement in overall survival at final analysis. Kisqali, in contrast, achieved a win in that setting in the Monaleesa-2 trial.
The data presented at ESMO showed that Verzenio in combination with endocrine therapy reduced the risk of death by 16%. The seven-year OS rate in the ITT population was 87% in the Verzenio arm compared with 85% for the control arm.
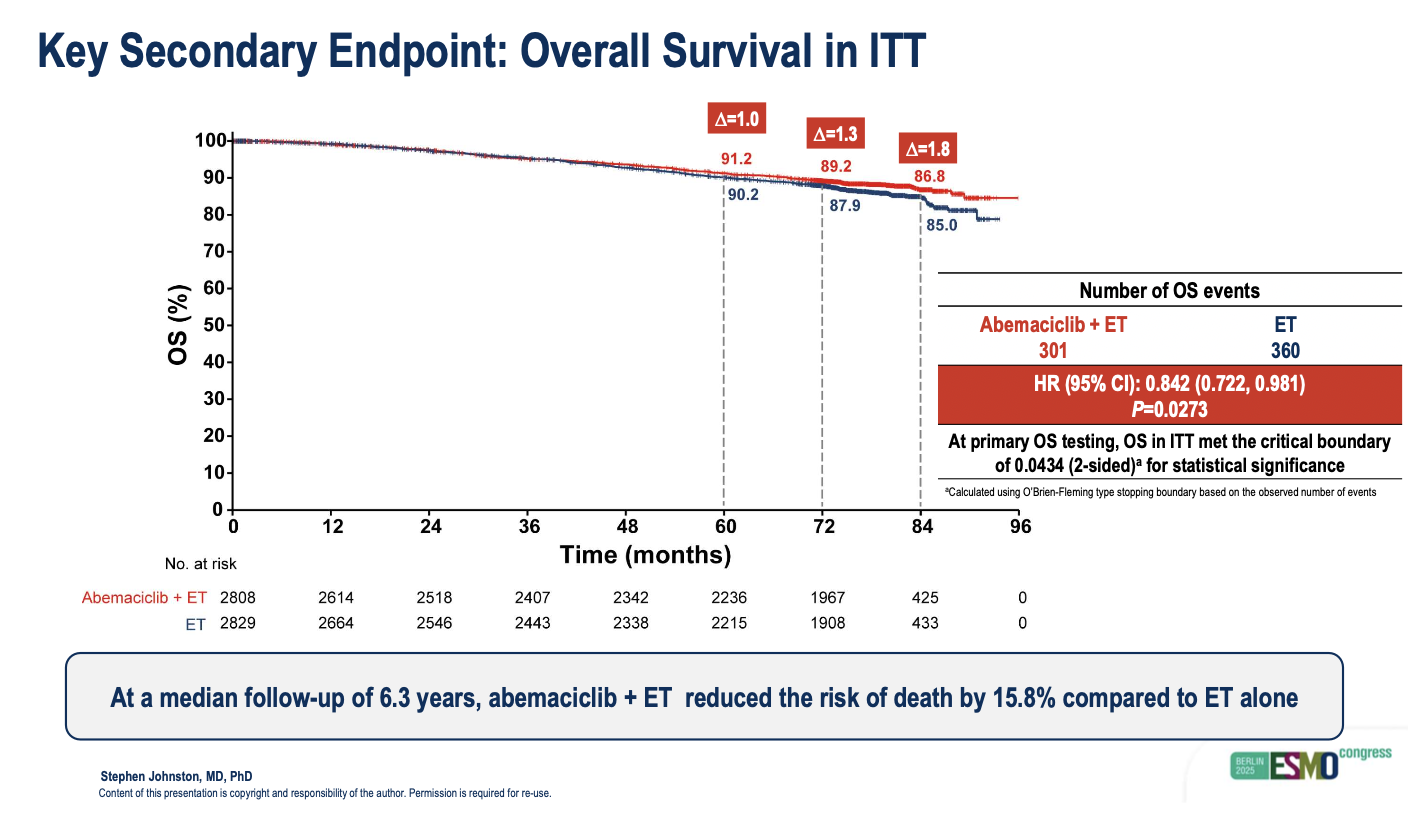
The iDFS numbers, meanwhile, continued to show a benefit for the Verzenio cohort. The seven-year iDFS rate reached 77% for patients treated with the CDK4/6 inhibitor, compared with 71% for control patients.
The overall survival data for Natalee are not expected any time soon, but it will be interesting to see if that trial can demonstrate a benefit with Kisqali not only in node-positive but also in node-negative patients.
1716













