
ESMO 2025 – Novartis looks for Pluvicto prostate Addition
But the ESMO discussant recommends against widespread use in hormone-sensitive disease.
But the ESMO discussant recommends against widespread use in hormone-sensitive disease.

Novartis, looking to expand its PSMA-targeting radiopharmaceutical Pluvicto into early prostate cancer, is heading to regulators, backed by a 28% reduction in the risk of disease progression or death in the PSMAddition trial in metastatic hormone-sensitive disease.
However, overall survival data were immature, ESMO heard during a presidential session on Sunday. And the ESMO discussant, Dr Arun Azad of Peter MacCallum Cancer Centre in Australia, poured doubts on Pluvicto’s chances of widespread use in this setting.
He said that while the radiographic progression-free survival benefit seen in PSMAddition would probably lead to regulatory approval, it was unlikely that an OS benefit would ever be seen, even with longer follow-up.
Novartis plans to file Pluvicto with the FDA in hormone-sensitive disease this year, based on final rPFS and updated OS results. It will hope for a swift path to market, particularly after the agent’s tribulations in pre-chemo, castrate-resistant prostate cancer, where it finally got US approval earlier this year, based on PSMAfore, following doubts about OS data in that trial.
One problem in PSMAfore was a high number of patients crossing over from control to Pluvicto. And crossover was also seen in PSMAddition, which compared Pluvicto plus androgen deprivation therapy (ADT) plus androgen receptor pathway inhibitor (ARPI), versus ADT plus ARPI, in PSMA-positive HSPC.
In PSMAddition, 16% of the control arm crossed over to Pluvicto – equating to 60% of the control patients who progressed.
Crossover was the reason behind Azad’s pessimism around the final OS result. He also noted that Pluvicto didn’t improve quality-of-life measures; indeed, one metric was numerically worse. Pluvicto was associated with side effects, particularly dry mouth and GI toxicity, and a risk of secondary malignancies.
Azad concluded that he’d only consider using Pluvicto in particularly severe hormone-sensitive disease.
New Addition?
The primary endpoint of PSMAddition was rPFS (radiographic progression-free survival), with OS a secondary endpoint. The data presented at ESMO come from an interim analysis, with a cutoff date of 13 January 2025.
Median rPFS wasn’t reached in either arm, but there were 139 events (24%) in the Pluvicto arm, versus 172 events (30%) in the control cohort, giving a 28% risk reduction with a p value of 0.002.
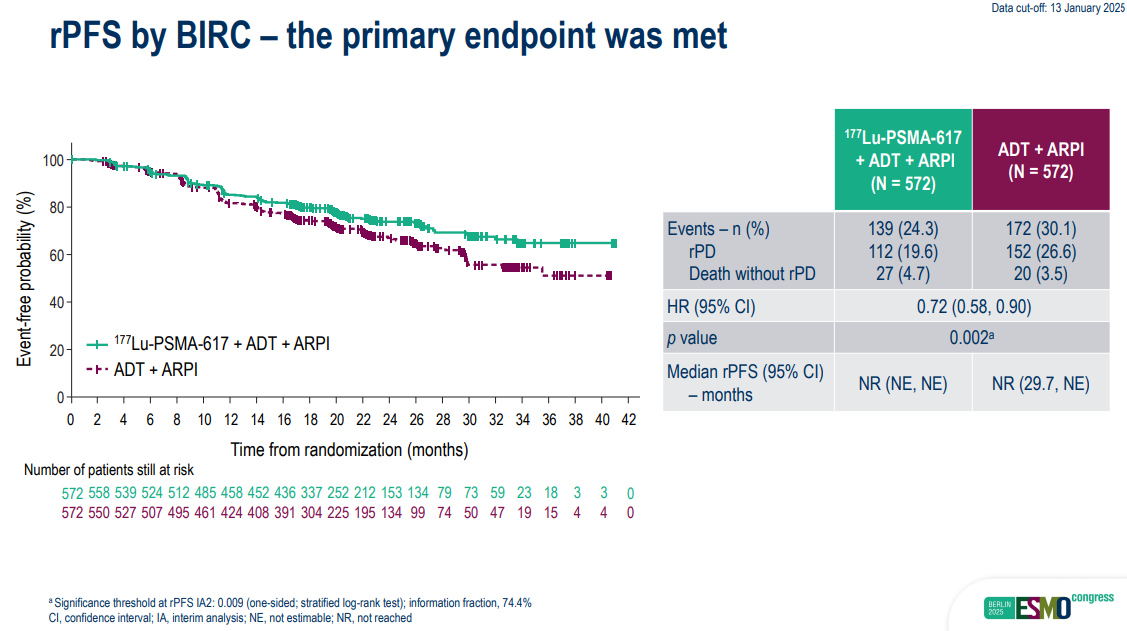
Source: Dr Scott Tagawa & ESMO.
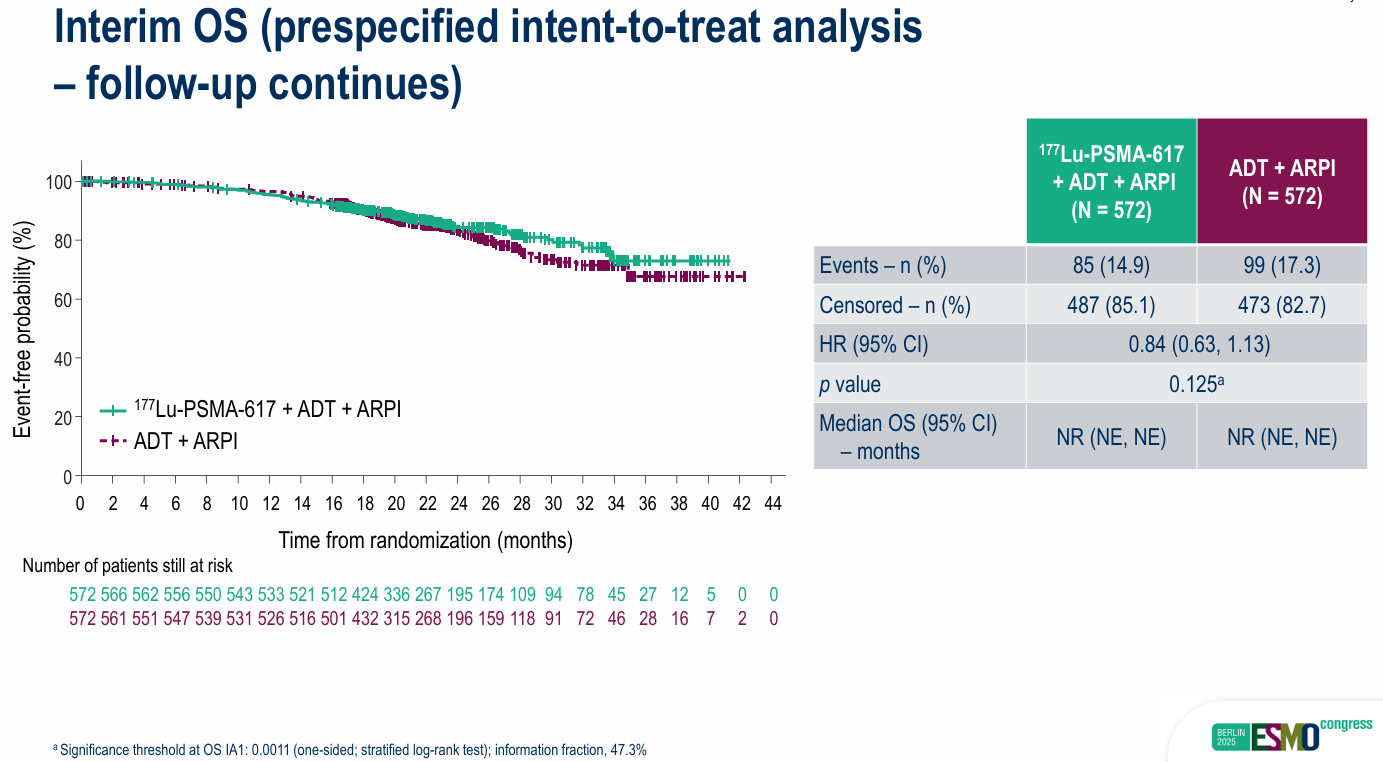
Meanwhile, Novartis claimed a positive OS trend based on the immature data. At the time of the analysis, around 15% of patients in the trial had died, representing 47% of the events needed to trigger the final OS analysis.
The OS hazard ratio was 0.84 – however, the upper bound of the confidence interval was above 1.
Novartis has forecast that Pluvicto, which sold $1.4bn in 2024, will bring in peak revenues of over $5bn. Approval in hormone-sensitive disease would be a big step towards that, by doubling the number of patients eligible for the drug. However, the ESMO session has raised questions about just how big the product might become.
1787













