
ESMO 2025 – Tubulis keeps the NaPi2b dream alive
The first TUB-040 data come just after Tubulis raised €308m.
The first TUB-040 data come just after Tubulis raised €308m.

As the only non-Chinese biotech still standing with a clinical-stage anti-NaPi2b conjugate in its pipeline Germany’s Tubulis has a lot to prove, but late-breaking data revealed at ESMO on Sunday suggest that it has a chance to succeed where the likes of Mersana and Roche failed.
A lot of private investor money is now riding on Tubulis, which shortly before ESMO closed a €308m series C financing round claimed to have been the biggest ever for a European biotech company. This came some 18 months after a €128m series B2 raise, and was said to have been targeted largely at development of the anti-NaPi2b ADC TUB-040, the subject of Sunday’s ESMO late-breaker.
The ESMO results concern 66 efficacy-evaluable platinum-resistant ovarian cancer patients, and represent the first ever human data publicly revealed for TUB-040. The headline number is a 50% confirmed response rate among 46 subjects given 1.67-3.3mg/kg doses.
The study is testing 0.5-5.3mg/kg, but the presenter, Dr Antonio González-Martín of University of Navarra, focused on 1.67-3.3mg/kg, which looks like a likely range for future study. There were no responses below 1.67mg/kg, and 4.4mg/kg was determined to be the maximum tolerated dose.
The results include one complete response, at 2.5mg/kg; there are an additional four unconfirmed responses, but it wasn’t apparent how many of these were ongoing and thus capable of being confirmed in future.
First-in-human data for TUB-040
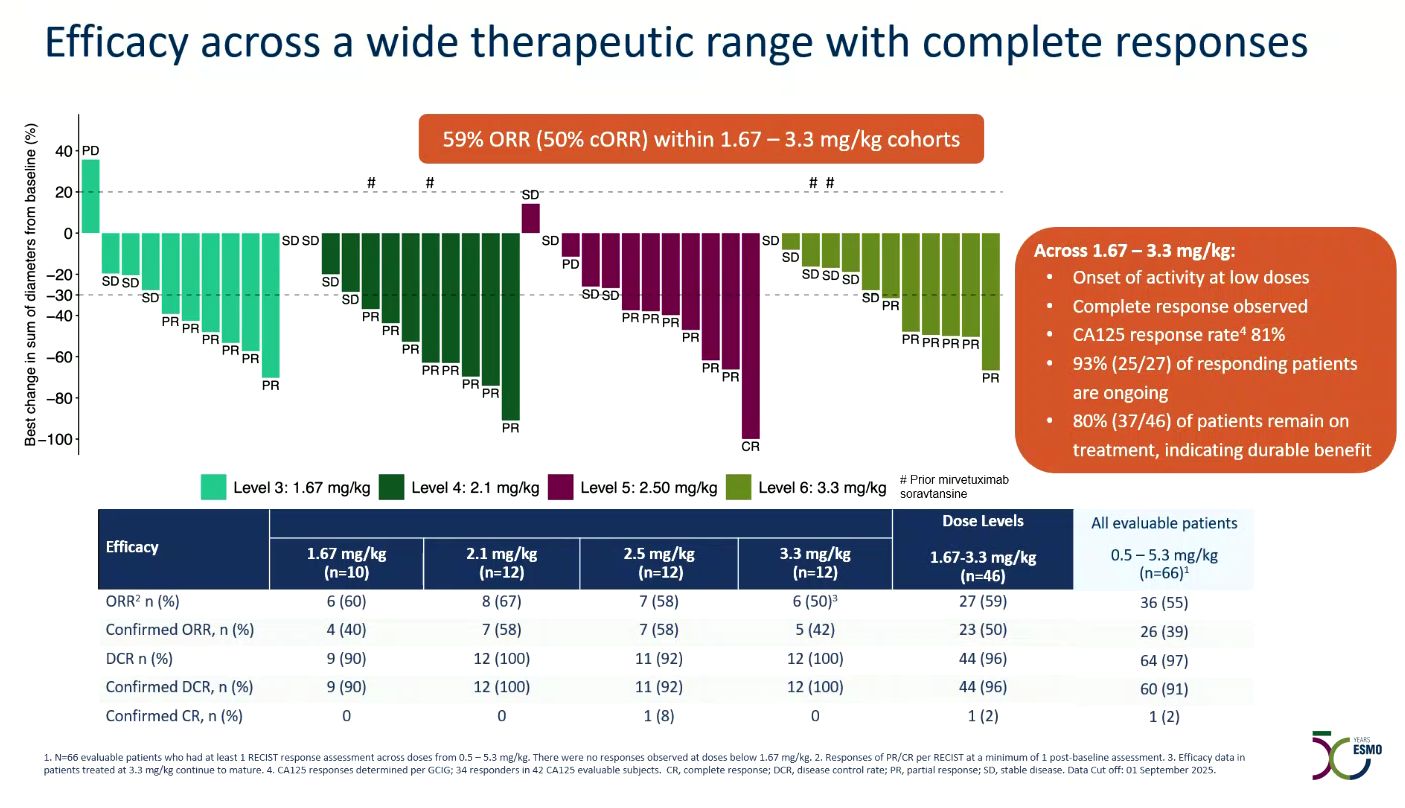
Not only is this level of activity unprecedented for a Napi2b-targeting ADC, TUB-040 was said to have a wide therapeutic window, with no clinically relevant bleeding, pneumonitis, ocular toxicity, stomatitis or neuropathy reported, something Tubulis says distinguishes TUB-040 from other topoisomerase 1 inhibitor-based ADCs.
There were no fatal treatment-emergent adverse events across all cohorts and, while two patients discontinued because of adverse events at any dose, there were no such discontinuations in the 1.67-3.3mg/kg range.
The difficulties of developing anti-Napi2b ADCs are illustrated not only by Mersana, which has scrapped not one but two clinical-stage assets, but also by discontinuations at Roche and Zymeworks, which earlier decided against taking ZW220 into clinical trials.
Alluding to such a poor precedent, González-Martín called the TUB-040 data a “completely new history in Napi2b”. TUB-040 uses an Fc-silent IgG1 antibody said to minimise off-target toxicity, and a linker designed to avoid premature payload loss, but it’s not clear whether Tubulis's success can be put down solely to such characteristics.
2748













