Huabo goes pivotal with bispecific VEGF trap
The company takes a PD-L1 x VEGF asset into phase 3.
The company takes a PD-L1 x VEGF asset into phase 3.

The PD-(L)1 x VEGF bispecific area continues to expand, with yet another molecule advancing into late-stage development. This time, the focus turns to the Chinese biotech Huabo Biopharm, after the posting of the first phase 3 study on clinicaltrials.gov for its PD-L1 x VEGF fusion protein sotiburafusp alfa.
Sotiburafusp alfa is a recombinant, humanised fusion protein built around an anti-PD-L1 monoclonal antibody fused to VEGFR-1. The architecture resembles palverafusp alfa, an asset Instil Bio walked away from earlier this year, but which ImmuneOnco has since committed to continue developing.
According to the registry entry, the new phase 3 trial, HB0025-C-0302, will evaluate sotiburafusp alfa in combination with chemotherapy head-to-head against Keytruda and chemo in the first-line squamous NSCLC setting, with PFS as the primary endpoint. Patients will be stratified by PD-L1 expression (<1%,1-49%, and ≥50%), and those with EGFR and ALK pathway mutations will be excluded.
Sotiburafusp is also notable mechanistically for hitting PD-L1, like BioNTech/Bristol Myers Squibb's pumitamig (and unlike Summit/Akeso's ivonescimab, which hits PD-1), and so likely relying on the principle of being anchored to PD-L1-expressing tumour cells.
Early ESMO splash
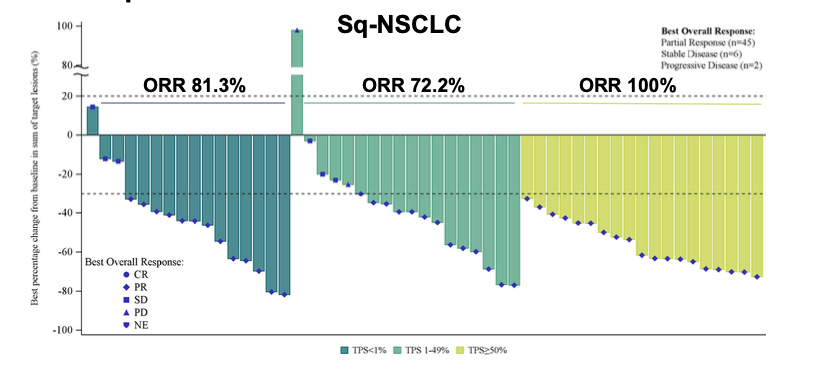
The pivotal trial is supported by eye-catching efficacy data presented by Huabo at last year's ESMO conference. In that presentation, the company reported a response rate of 84% in 62 treatment-naive patients with squamous NSCLC treated with sotiburafusp alfa, climbing to 100% in patients with PD-L1 expression of at least 50%, although all responses observed were partial.
Safety will be a key point to watch, however. Grade ≥3 treatment-related adverse events occurred in 40% of patients, and Huabo disclosed that two patients died owing to a treatment-related adverse event.
The lung cancer results build on similarly strong data in endometrial cancer, where Huabo reported an 84% response rate at ASCO. Although the company announced plans at the time to start a dedicated endometrial cancer trial, that study has yet to appear on clinicaltrials.gov.
Huabo’s advance comes as BioNTech has posted a new phase 3 trial of pumitamig in first-line lung cancer, Rosetta-Lung202, focusing exclusively on patients with PD-L1 expression of 50% or higher; nine pivotal trials of the PD-L1 x VEGF bispecific are now listed on ct.gov, with another planned in first-line head and neck cancer.
Beyond sotiburafusp alfa and palverafusp alfa, another company is pushing the fusion protein concept. Doer Biotechnology is developing DR30206, a triple target fusion protein designed to inhibit PD-L1, TGF-β and VEGF. The asset is being evaluated in a phase 3 trial in gastric cancer in China, alongside a phase 1/2 study in solid tumours.
Sotiburafusp alfa ORR data in first-line squamous NSCLC
25