Nkarta plans its comeback
The company puts its faith in a new lymphodepletion regimen to overcome mediocre early results with its lead Car-NK therapy.
The company puts its faith in a new lymphodepletion regimen to overcome mediocre early results with its lead Car-NK therapy.

Just like Fate Therapeutics, Nkarta found an initial NK cell therapy breakthrough to have been short-lived, data presented today have revealed. It has emerged that the latter group had to go back to the drawing board to redesign its chemo conditioning regimen to give a product that it now hopes will be more efficacious and longer-lasting.
Now emerge the first results of this new approach, as used with Nkarta’s lead pipeline asset, NKX101: four of six late-line acute myelogenous leukaemia patients have been put into remission, the company said today. Though this seems promising, however, investors might sense echoes of last year’s false dawn, and could come away asking to see more data.
Nkarta’s valuation is pertinent here: at $225m this resolutely refuses to approach the market cap of Fate – in spite of Fate’s 60% collapse in January as that group purged its NK cell therapy pipeline, cut jobs and revealed the loss of Johnson & Johnson as a deal partner.
Illusory
It was just over a year ago that Nkarta had boasted of a major clinical success with NKX101, an allogeneic NK cell therapy expressing a Car against NKG2D ligands in AML, reporting five remissions among 17 patients, and raising $230m on the strength of the data.
Given the aggressive and intractable nature of AML this might have been respectable enough, but Nkarta talked up five subjects given the highest NKX101 doses, 1-1.5 billion cells spread over three infusions. Three of these five had gone into compete remission, and the company moved to treat more patients with this dosing regimen.
But things then went quiet, and today investors found out why: an additional 13 high-dose patients have yielded just one new response – a complete remission with residual thrombocytopaenia. Not only that, but NKX101 had no durability: of the three initial responders, two relapsed within a couple of months, while the third was transplanted.
If this makes it clear that the initial promise of high doses was illusory, Nkarta has moved quickly to avoid the trap Fate fell into. A new lymphodepletion regimen has been investigated, substituting flu/cy’s cyclophosphamide element with cytarabine, a chemotherapy that Nkarta claims upregulates NKG2D ligands on blast cells.
Importantly, alongside the postmortem on the initial dataset, today brought the first clinical efficacy data of such flu/cytarabine preconditioning with NKX101, given as 1.5 billion cells over three doses: results with six patients show three going into full remission, and a fourth reporting complete response with residual thrombocytopenia.
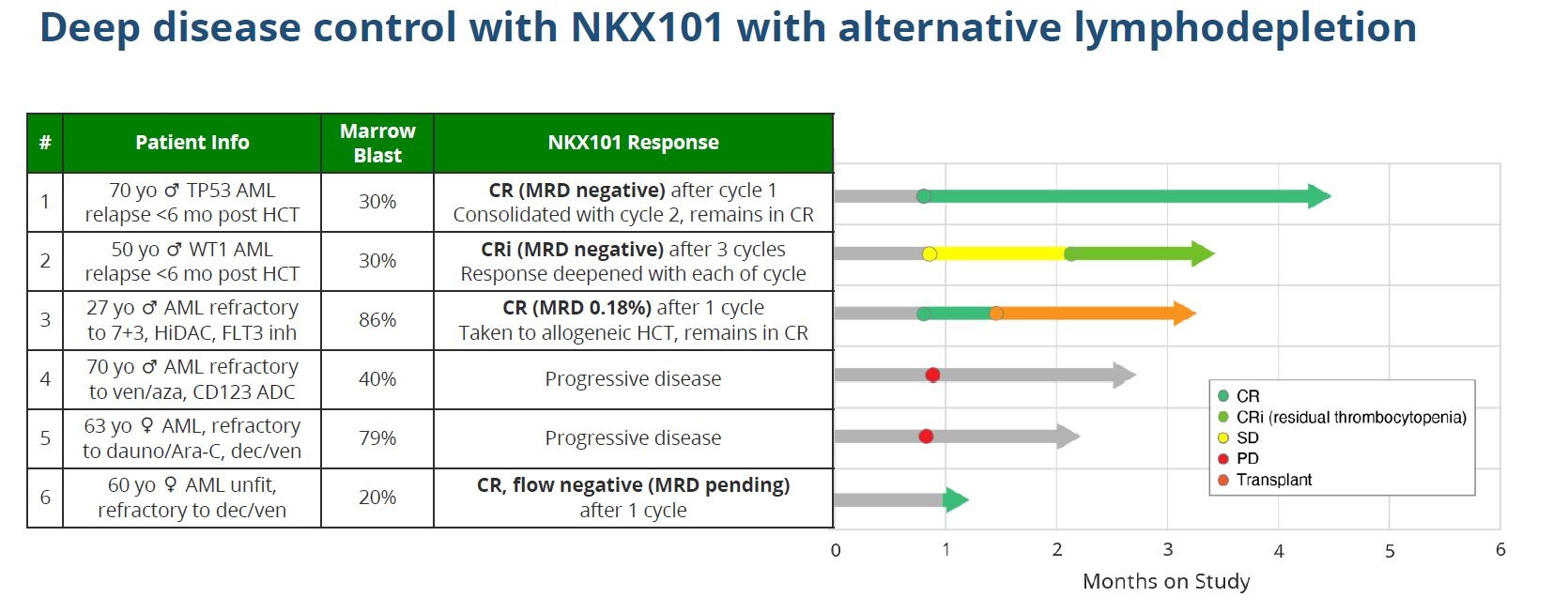
Flu/cytarabine is lymphodepletion approach Nkarta will now use with NKX101, but as cytarabine is particularly well suited in AML it will for now not be used with the group’s other projects.
Nkarta also highlighted the fact the new approach resulted in no cytokine release or neurotoxicity of any grade, though half the patients reported grade 3 sepsis. Dosing NKX101 with flu/cyclophosphamide lymphodepletion yielded 12% mild cytokine release, and 60%, 53% and 43% respective rates of severe thrombocytopenia, anaemia and neutropenia.
While these data do look promising, there is a caveat. Most importantly, Nkarta cautions that follow-up from the six patients is limited, and only one of the four responders appears to have been in remission for over a month. Like the April 2022 data, the latest update might be a false dawn.
Fate’s experience suggested that NK cells do not expand like T cells, meaning that it might be impossible to dose a product high enough, and this will weight heavy. Nkarta is not planning to update the flu/cytarabine NKX101 cohort until the first half of 2024, so until then investors will just have to sit tight.
492