
ASCO 2025 – Astra seeks a new SERD paradigm
But camizestrant’s use could depend on uptake of monitoring – for now.
But camizestrant’s use could depend on uptake of monitoring – for now.

AstraZeneca, seeking an earlier use for its oral SERD camizestrant, told ASCO on Sunday how a new paradigm of treating patients who develop the ESR1 mutation during first-line therapy but before progression improves not only progression-free survival but also PFS2, a more relevant real-world endpoint.
However, the real test will be overall survival, and these data are still immature. And this monitoring strategy will require a shift in mindset among doctors, which could take time, Astra admitted during a pre-ASCO media event. The company expects camizestrant data next year in a more conventional first-line setting, from the Serena-4 trial that, if successful, could negate the need for monitoring.
Meanwhile, the group’s head of oncology R&D, Susan Galbraith, confirmed that a second-line label for camizestrant was no longer a focus, despite the project trumping other SERDs by showing a benefit in all comers in the phase 2 Serena-2 study.
First-line focus
Astra has always felt that the biggest impact for camizestrant would come in earlier breast cancer lines, Galbraith added.
Serena-6 is one of these early efforts. The study enrolled first-line ER-positive, HER2-negative patients who'd received standard treatment with CDK4/6 and aromatase inhibitors for at least six months, and monitored them for development of ESR1m every two to three months, using ctDNA analysis as part of routine tumour scanning. Patients in whom ESR1m was detected – but who hadn’t had disease progression by imaging – were then given camizestrant plus the CDK4/6 inhibitor, or continued on their original therapy.
At ASCO Astra disclosed that median PFS in the camizestrant switch group was 16.0 months, versus 9.2 months in the control group. This represented a 56% reduction in the risk of disease progression or death, with a p value of less than 0.00001.
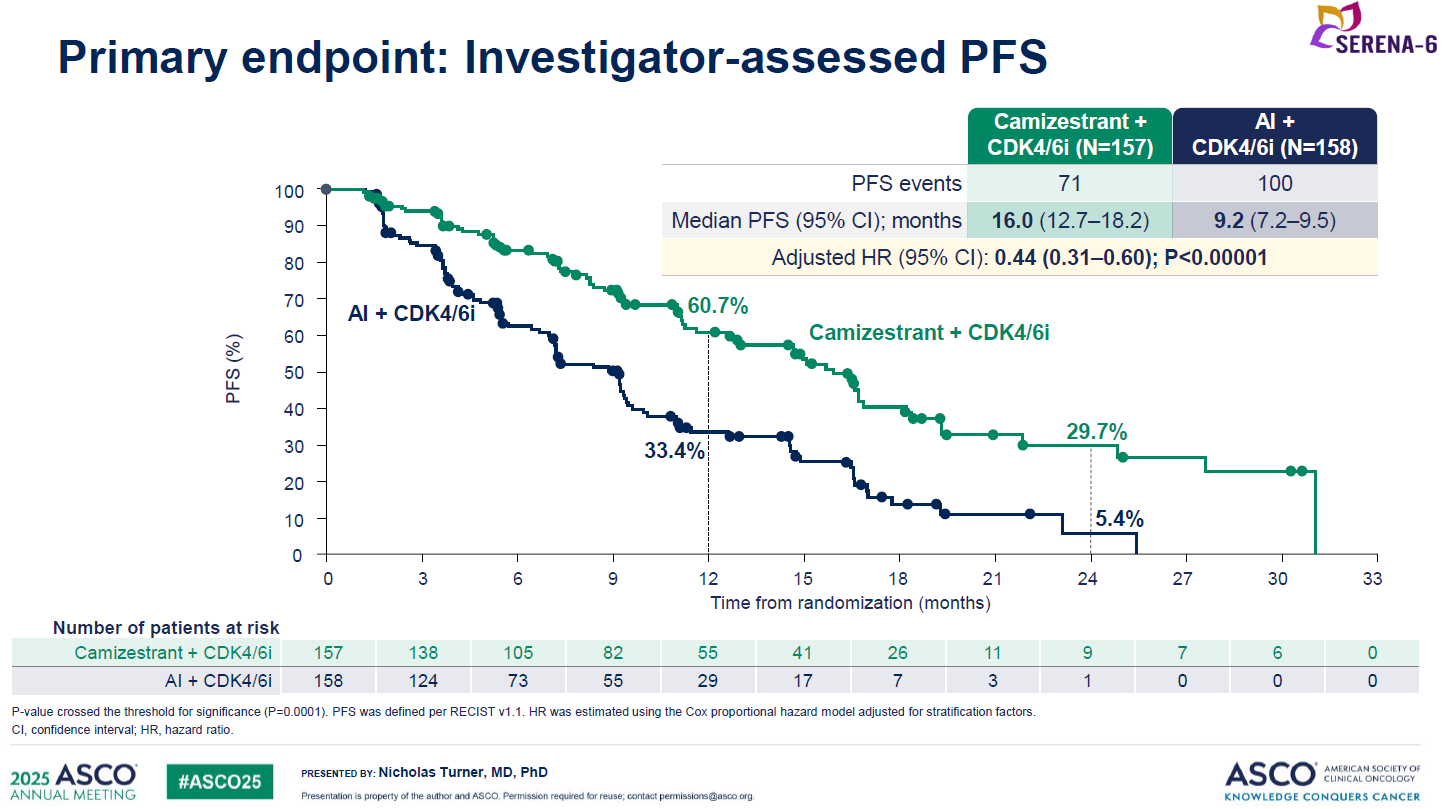
Galbraith also highlighted a benefit on PFS2, a secondary endpoint that measures time from randomisation to progression on a second-line therapy, with a hazard ratio of 0.52. This is relevant because PFS benefit alone will count for little if patients who progress via ESR1 resistance can then simply be given a second-line SERD like Orserdu, with no detriment to overall survival.
OS data in Serena-6 are still “very immature”, and no OS data were presented at ASCO, although Galbraith said the PFS2 results were “encouraging” for a future positive OS trend. However, she also seemed to dial down expectations of a significant OS benefit, noting that Serena-6 had a “relatively small number of patients” and that OS powering wasn’t the same as for PFS.
While Astra hasn’t yet disclosed a regulatory submission for camizestrant based on Serena-6, its chief executive, Pascal Soriot, said “with data like these we’d rush to file”.
Learning curve
If camizestrant is approved the next hurdle could be getting doctors on board with the new monitoring paradigm. Dave Fredrickson, head of Astra’s oncology haematology business, noted that ESR1m testing was widely available, but conceded that there would be a “learning curve” in terms of implementing this use.
Still, a win in the Serena-4 study could avoid this complication entirely. That trial tests first-line camizestrant plus the CDK4/6 inhibitor Ibrance, versus Ibrance plus the aromatase inhibitor anastrazole, with a primary endpoint of PFS.
After all, if patients can be treated first line regardless of ESR1m status, that would remove the need for testing. Primary completion, according to clinicaltrials.gov, is August 2026 – suggesting data could come towards the end of next year.
In the meantime, another big SERD first-line readout is due this year, from Roche’s Persevera trial of giredestrant.
Astra is also going even earlier, testing camizestrant in adjuvant settings in the Cambria-1 and 2 trials.
2607













