
ESMO 2025 – more ivonescimab shots on goal for Summit
Harmoni-6 succeeds in first-line lung cancer, but the focus is on changes to Harmoni-3.
Harmoni-6 succeeds in first-line lung cancer, but the focus is on changes to Harmoni-3.

An ESMO presidential session on Sunday confirmed the promise of Akeso/Summit’s anti-PD-1 x VEGF bispecific ivonescimab, backing a topline win in the Chinese Harmoni-6 lung cancer study with progression-free survival numbers. This sets the stage for a much bigger test – whether such a success can be repeated in the US Harmoni-3 trial, against Merck & Co’s Keytruda rather than BeOne’s Tevimbra.
As such, Summit investor focus has turned to Harmoni-3, and the fact the company has just made important changes to this key US study to give itself more chances to succeed. Harmoni-3 has a similar design to Harmoni-6, testing the bispecific chemo versus PD-1 plus chemo, but its comparator is Keytruda rather than Tevimbra, and it includes non-squamous disease.
Like Harmoni-6, Harmoni-3 enrols first-line NSCLC patients irrespective of PD-L1 status, and asks whether the addition to chemo of a bispecific PD-1 x VEGF approach is better than adding PD-1 blockade alone. Harmoni-3 had started out as a 400-patient trial testing a single OS primary endpoint, and enrolling only patients with squamous NSCLC (the same histology as in Harmoni-6).
Changes, and more changes
Last year, however, the decision was made to add the non-squamous histology, and to promote PFS to a co-primary endpoint – still in the all-comers population. This addition, and the need to maintain sufficient powering for a dual primary endpoint, resulted in the enrolment target being more than doubled.
Now comes another change, with Summit revealing that the PFS and OS co-primaries are to be split further, to test the benefit in squamous and non-squamous patients separately. The number of patients is going up again, with a planned 600 patients with squamous and 1,000 patients with non-squamous NSCLC now to be enrolled into Harmoni-3.
One benefit is that this might allow an earlier readout to this trial, which earlier wasn’t expected to yield data until 2027; Summit now expects the squamous cohort to hit enough events to allow a PFS readout in the second half of next year, at which point an OS analysis might be performed too. For non-squamous NSCLC PFS data will come in the first half of 2027.
Importantly, however, the change has also bought Summit additional shots on goal. It’s now possible for ivonescimab to succeed statistically in one histology but not the other, and the prominence of PFS as a primary endpoint might take the heat off the need to demonstrate an especially strong OS benefit.
How Harmoni-3 has evolved
| Histology | Enrolment target | Primary endpoint | |
|---|---|---|---|
| Jun 2023 | Squamous | 400 | OS |
| Dec 2024 | Squamous & non-squamous | 1,080 | OS & PFS |
| Oct 2025 | Squamous & non-squamous | 1,600 | OS & PFS in squamous OS & PFS in non-squamous |
Source: OncologyPipeline & Summit statement.
Whether ivonescimab can ever beat Keytruda on OS remains a key unanswered question, not only in Harmoni-3 but in other trials too. And the Harmoni-6 data don’t help answer it either, given that the ESMO presentation was on PFS, with OS said to be immature.
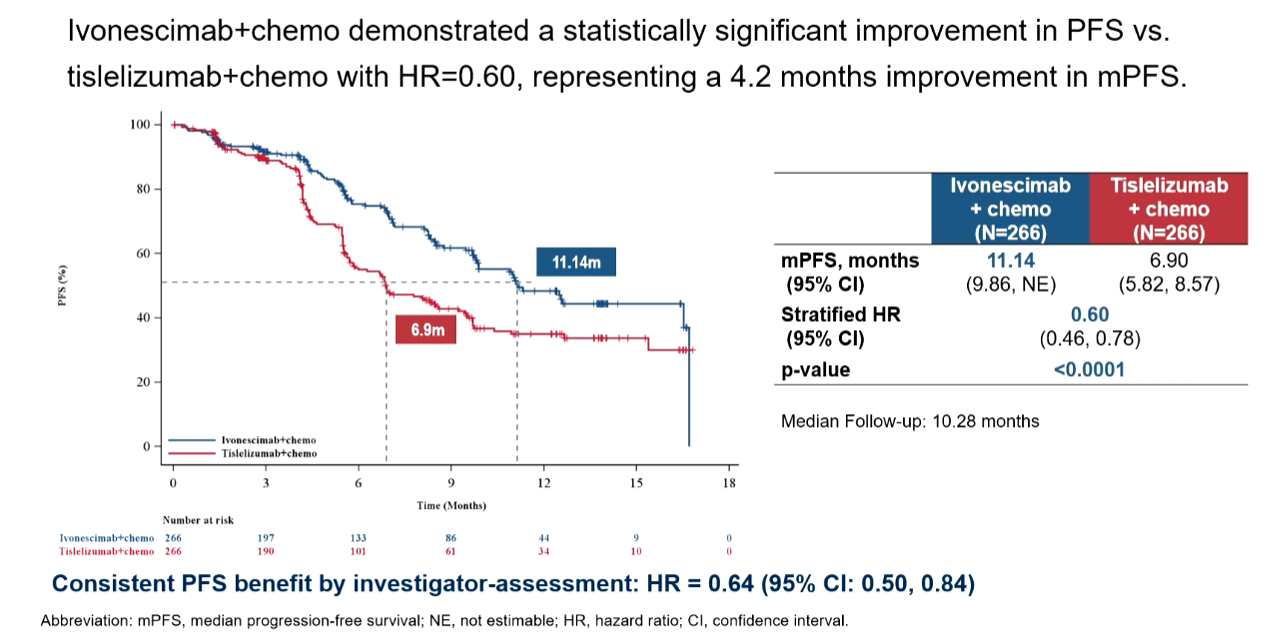
With the Harmoni-6 data having been toplined as "statistically significant and clinically meaningful" for PFS in April the focus at ESMO was on the magnitude of the benefit over Tevimbra plus chemo, and this was revealed as just over four months, with a PFS hazard ratio of 0.60 hitting a p value below 0.0001. This comfortably met Evercore ISI analysts’ expectations ahead of the readout.
The control cohort’s mPFS benefit of 6.9 months for Tevimbra plus chemo was slightly below the 7.7 that this combo scored in the Chinese registrational Rationale-307 study in first-line squamous NSCLC. A baseline for Summit to beat in Harmoni-3’s squamous cohort is 6.4 months of mPFS scored by Keytruda plus chemo in Keynote-407, though this readout is now rather old.
It was especially promising that Harmoni-6’s PFS benefit didn’t seem to be affected by patients’ PD-L1 status: the PFS hazard ratio in PD-L1 non-expressers was an impressive 0.55.
The only revelations about OS, however, were that statistical alpha was split, necessitating the clearance of a one-sided p value of 0.025, and that powering assumed a reduction in risk of death of at least 27%. These may or may not be relevant to understanding the design of Harmoni-3.
Harmoni-6's primary endpoint: PFS by blinded independent review
3313













