
BioNTech’s big gotistobart day approaches
Readout of the Preserve-003 trial in post-PD-(L)1 NSCLC is due in December.
Readout of the Preserve-003 trial in post-PD-(L)1 NSCLC is due in December.

The jury is still out on whether BioNTech’s gotistobart represents a relatively safe way of targeting CTLA-4, but the company will soon have phase 3 data that could make or break this asset.
Results from the Preserve-003 trial, in post-PD-(L)1 non-small cell lung cancer, are due at the North America Conference on Lung Cancer on 6 December. This study, originally in squamous and non-squamous patients, went on clinical hold in October 2024; that hold was lifted two months later, but the trial continued in squamous patients only.
At the time of the hold, BioNTech noted that the Preserve-003 had shown “varying results” between the squamous and non-squamous populations, explaining the reason behind the change. NACLC could show the data behind the decision, and also whether there was any imbalance in toxicity between the two disease histologies.
The results will come from stage one of the study, which tested gotistobart at 3mg/kg or 6mg/kg every three weeks, versus docetaxel, in both squamous and non-squamous patients.
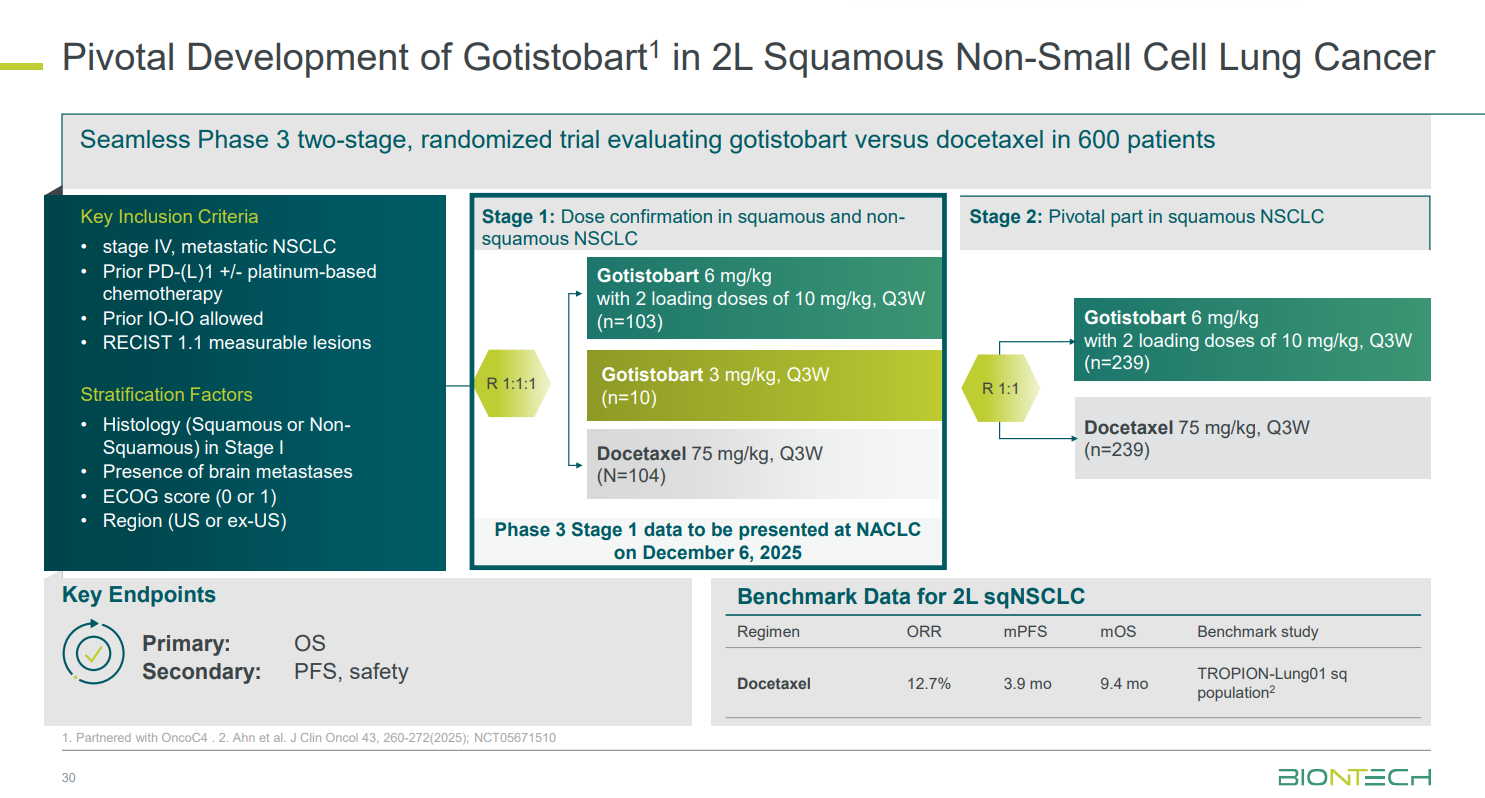
BioNTech set out its expectations at its recent R&D day. The bar to beat here comes from the control arm of Tropion-Lung01, which in the squamous population found an ORR of 13%, a median PFS of 3.9 months, and a median OS of 9.4 months with docetaxel.
Early evidence suggests that gotistobart could clear this hurdle. BioNTech cites an ORR of 30% with gotistobart among 27 checkpoint inhibitor-resistant NSCLC patients in the Preserve-001 phase 1 basket trial. However, two of eight responses were unconfirmed; excluding these gives an ORR of 22%.
Tropion-Lung01, meanwhile, failed to show a benefit with AstraZeneca’s TROP2-targeting ADC Datroway in squamous disease, and although it succeeded in the non-squamous population, Astra abandoned this use in favour of second-line EGFR-mutated NSCLC.
BioNTech therefore has a chance to move gotistobart into an underserved niche. The German group is already recruiting into the second stage of Preserve-003, testing gotistobart 6mg/kg versus docetaxel in squamous patients only. Results are expected in 2026.
Less toxic?
While efficacy data from Preserve-003 will be important, investors will also be keeping an eye on toxicity. Gotistobart, an anti-CTLA-4 MAb BioNTech licensed from OncoC4 for $200m, is claimed to have a smart design, using a pH-sensitive molecule to avoid autoimmunity-related side effects that have dogged the likes of Bristol Myers Squibb’s Yervoy.
But it’s unclear whether gotistobart is indeed any safer. It’s notable that Preserve-003 uses a 3mg/kg or 6mg/kg dose, while dosing in other studies has been reduced to 1mg/kg and 2mg/kg following toxicity.
Despite this, the phase 2 Preserve-004 trial in platinum-resistant ovarian cancer found a high rate of grade 3 or higher adverse events (55-72%) and discontinuations (21-28%) at 1mg/kg and 2mg/kg, the 2024 ESMO meeting heard.
However, Preserve-004 tested gotistobart plus Keytruda, while the upcoming Preserve-003 is evaluating gotistobart monotherapy. It should soon become apparent whether this will make any difference in terms of adverse events.
NACLC takes place on 5-7 December in Chicago.
3192













