
ASH 2025 – BeOne assuages sonrotoclax safety concerns
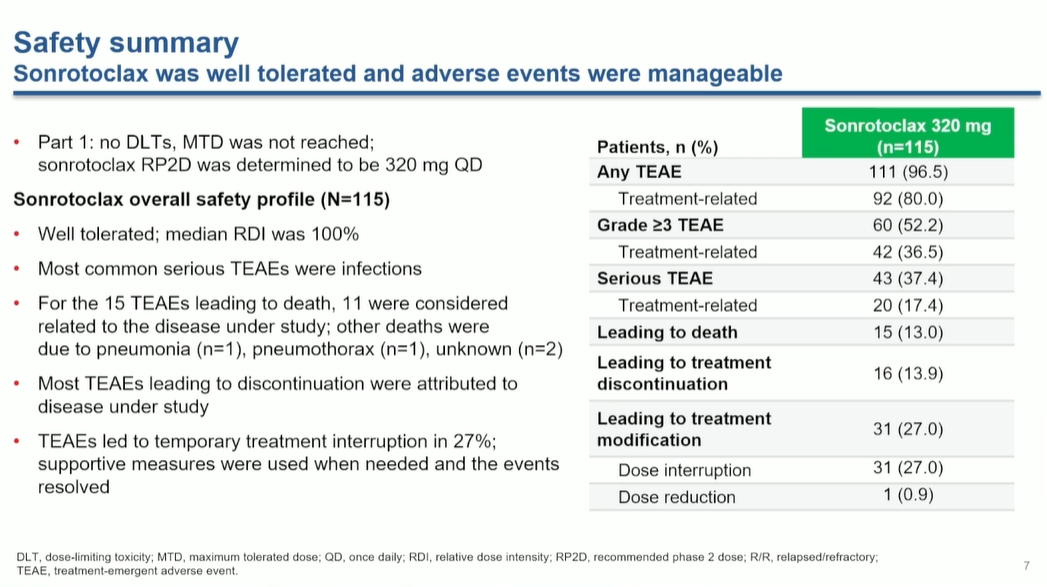
Of 15 treatment-emergent adverse events, only one was deemed related to therapy.
Of 15 treatment-emergent adverse events, only one was deemed related to therapy.

BeOne raised eyebrows in the build-up to ASH when the uncontrolled BGB-11417-201 trial of its next-gen BCL2 inhibitor sonrotoclax reported 15 treatment-emergent deaths, but full data from the study look likely to assuage concerns.
Of the 15 fatalities, 11 were due to progression of the disease in question, mantle cell lymphoma – and only one, caused by pneumonia, was deemed related to sonrotoclax, ASH heard on Sunday. Two of the remaining three deaths were of unknown causes, while one was down to pneumothorax.
BeOne’s chief medical officer, Amit Agarwal, noted in a pre-ASH interview with ApexOnco that BGB-11417-201, in post BTK-inhibitor MCL, enrolled very heavily pretreated patients, with a median of three prior lines of therapy. Still, reporting deaths due to disease progression as treatment-emergent adverse events seems very unusual.
Meanwhile, BGB-11417-201 reported a 52% ORR among 103 patients receiving a 320mg dose of sonrotoclax, consistent with the abstract. Median duration of response was 15.8 months, median progression-free survival was 6.5 months, and median overall survival wasn’t reached.
The result exceeded a 30% ORR seen with historical control, according to MD Anderson’s Dr Michael Wang, presenting the data. However, a more relevant benchmark now might be Lilly’s non-covalent BTK inhibitor Jaypirca, which has produced a 50% ORR in post-BTKi MCL, but a less impressive median duration of response of 8.3 months, BeOne's Agarwal pointed out.
Venclexta, AbbVie’s approved BCL2 inhibitor, isn’t approved for MCL; sonrotoclax was designed to be more potent than Venclexta.
BeOne is now awaiting an FDA decision for sonrotoclax in post-BTK inhibitor MCL, recently accepted with priority review. It has already begun the confirmatory Celestial-RR MCL trial, testing sonrotoclax plus BeOne’s BTK inhibitor Brukinsa, versus Brukinsa alone, in relapsed/refractory MCL patients who are BTK inhibitor naive but have previously received an anti-CD20 MAb.
The most important use for sonrotoclax, however, remains chronic lymphoblastic leukaemia, where phase 3 studies are ongoing in first and second-line settings – both including Venclexta as head-to-head comparator.
2225













