
SABCS 2025 – Gilead hasn’t given up on its Ascent
The company leaves the door open for Trodelvy pre-chemo, despite Ascent-07’s failure.
The company leaves the door open for Trodelvy pre-chemo, despite Ascent-07’s failure.

Gilead previously toplined the failure of Ascent-07, testing its TROP2-targeting ADC Trodely in pre-chemo breast cancer, and full data presented at SABCS on Wednesday showed this to be definitive. On the primary endpoint of centrally assessed progression-free survival, Trodelvy and control produced an identical median, of 8.3 months.
Still, it might not be the end, with Gilead buoyed by early overall survival numbers. Here, there was an early trend in favour of Trodelvy, with a hazard ratio of 0.72. When asked about future plans, a company spokesperson told ApexOnco: “When it’s appropriate, we will potentially engage regulators.”
However, they stressed that any OS analysis would be merely descriptive, given that the study didn’t meet its primary endpoint.
Gilead wasn’t any more specific about its next steps, only saying it would “share an update when it’s available”.
Hard Ascent
Trodelvy is currently FDA approved for late-line ER-positive, HER2-negative breast cancer, based on the Tropics-02 trial in patients previously treated with endocrine therapy and at least two additional drugs, including chemo.
Ascent-07 aimed to expand use into patients who had previously received endocrine therapy, but not chemo; the study tested Trodelvy versus investigator’s choice of chemo.
On the primary endpoint of PFS by blinded independent central review, median PFS in both groups was 8.3 months, with a hazard ratio of 0.85 and a non-significant p value of 0.13.
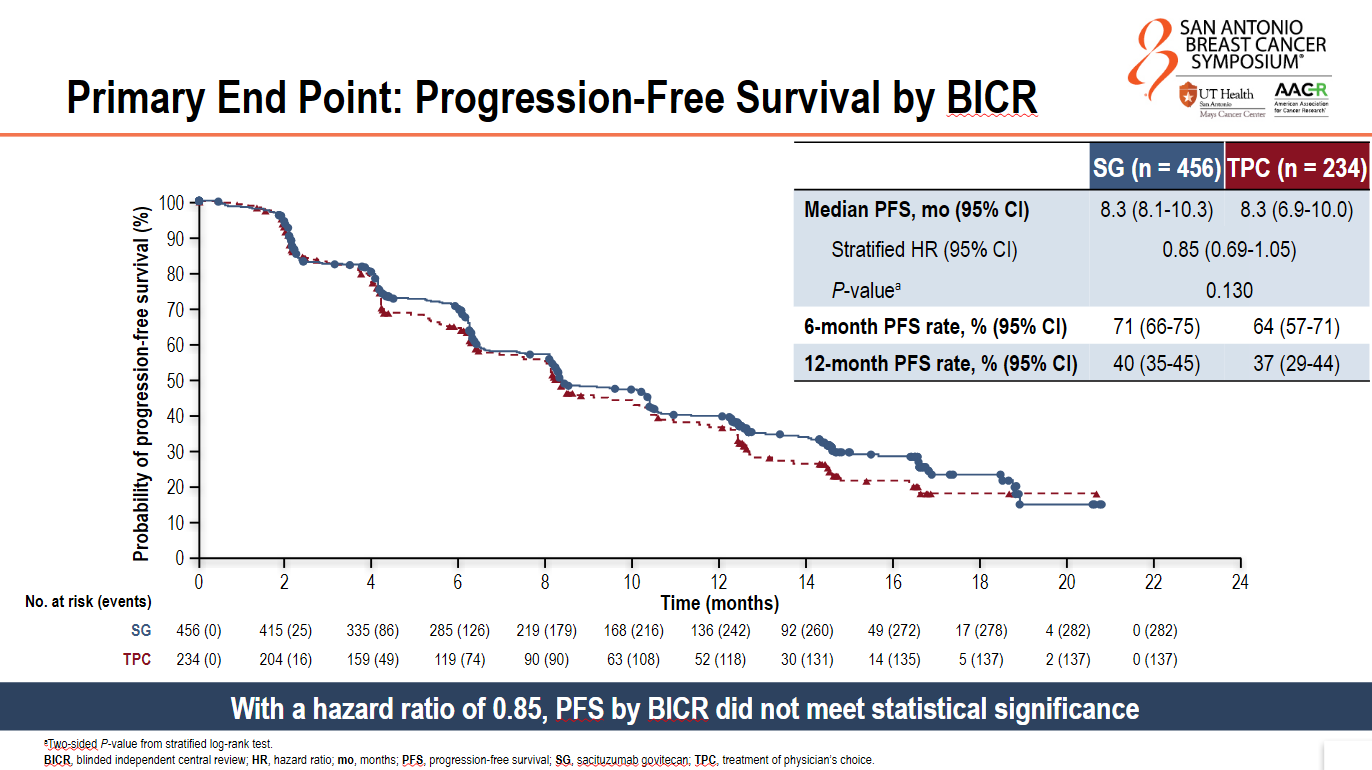
Investigator-assessed PFS looked more positive, with a hazard ratio of 0.78. Presenting the data at a SABCS press conference, Memorial Sloan Kettering’s Dr Komal Jhaveri blamed uneven censoring for the discordance, with more patients censored in the control versus the Trodelvy arm. The main reason for this was patients starting a new anticancer therapy, including another ADC.
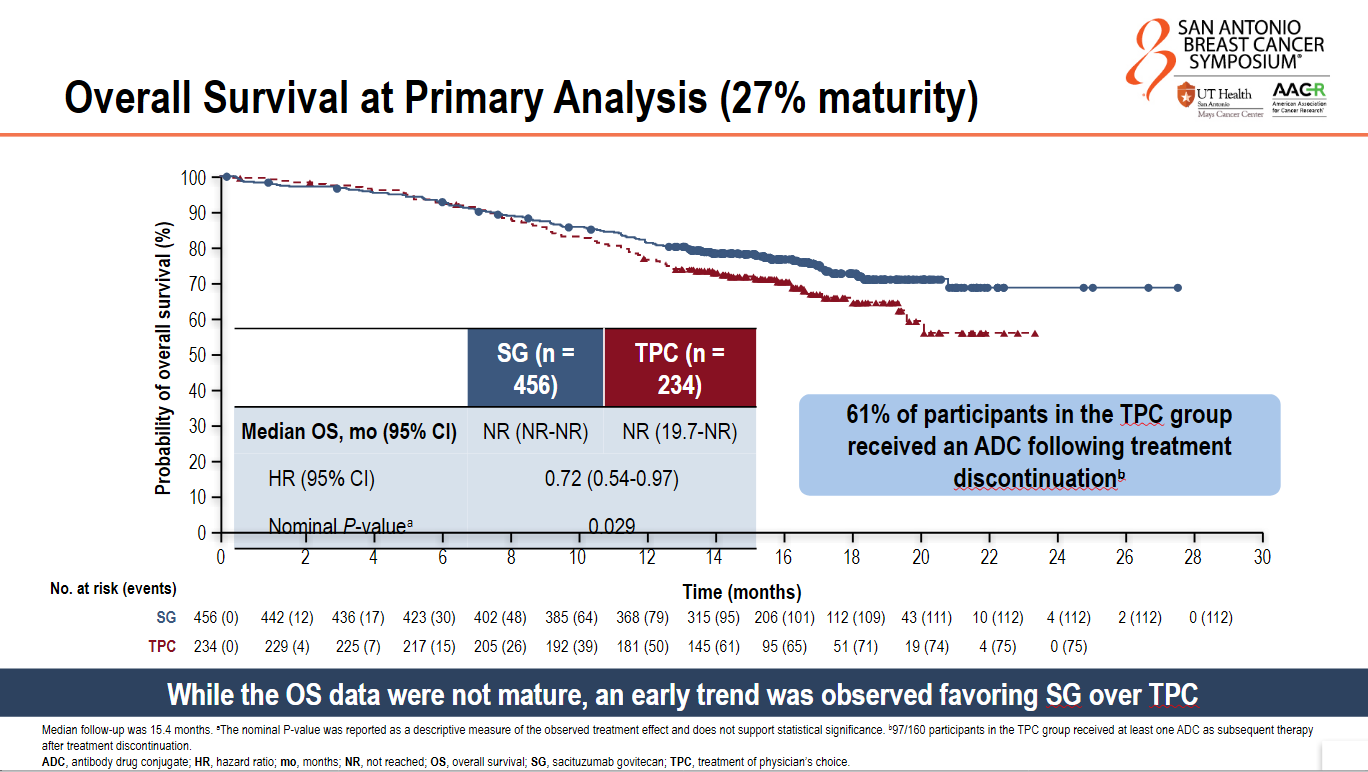
An early overall survival cut also showed an early trend favouring Trodelvy. At 27% data maturity the OS hazard ratio was 0.72 and the nominal p value was 0.029. Jhaveri described this trend as "interesting", noting that it came despite the fact that 61% of patients in the control arm went on to receive an ADC following treatment discontinuation.
The study will continue to assess overall survival.
Discussing the results, Dr Kate Lathrop of UT Health San Antonio suggested that one way forward might be ascertaining whether certain patients, such as those with higher TROP2 expression, did better on Trodelvy.
Jhaveri said her group would be “looking at activity and efficacy by TROP2 expression levels”, and hoped to present those data at a future meeting.
AstraZeneca and Daiichi’s TROP2 ADC Datroway is also FDA approved after endocrine therapy and chemo. That drug recently went into a pre-chemo phase 3, Tropion-Breast06, but that study doesn’t include a control arm.
And Merck & Co also has a pre-chemo trial ongoing with its rival project, sacituzumab tirumotecan. Trofuse-010 is testing saci-T, with or without Keytruda, versus physician’s choice of chemo, with completion set for 2027.
2237













