AbbVie's telisotuzumab conjugate take two
A phase 2/3 study might show whether teliso-A can do better than teliso-V.
A phase 2/3 study might show whether teliso-A can do better than teliso-V.

It seems as though AbbVie's efforts with Emrelis will, for now, go no further than its recently approved setting of second-line, cMet-high lung cancer. Attention is switching to telisotuzumab adizutecan, a follow-up cMet-targeting ADC that will soon start a phase 2/3 Tagrisso combo trial; this is a similar setting to the one in which an Emrelis pivotal study was scrapped last year.
The M25-287 study will compare teliso-A plus Tagrisso versus Tagrisso alone in first-line EGFR-mutant NSCLC patients, and begin in September, a new clinicaltrials.gov listing reveals. This appears to be an attempt to take teliso-A into earlier-stage NSCLC than Emrelis's approved use, in addition to AbbVie pursuing colorectal cancer, a setting not being targeted with Emrelis.
Teliso-A and Emrelis are both based on the MAb telisotuzumab, but the former is conjugated to a topoisomerase 1 inhibitor payload, while the latter uses MMAE (vedotin). Emrelis received US accelerated approval last month for pretreated cMet-positive NSCLC, but only in patients with high cMet overexpression, defined as ≥50% of tumour cells with strong (IHC 3+) staining.
In October 2023 AbbVie revealed plans to start M22-142, a phase 3 trial comparing Emrelis plus Tagrisso versus chemo in 250 EGFRm lung cancer patients with cMet overexpression, who had progressed on a third-generation tyrosine kinase inhibitor like Tagrisso. But this was abandoned last year for "strategic considerations", according to clinicaltrials.gov, with no patients enrolled.
Next up, teliso-A
Now comes M25-287, the new teliso-A plus Tagrisso trial, whose key difference versus the abandoned study is that it will enrol front-line patients. Moreover, though patients must consent to cMet expression analysis, there is no entry requirement for any level of cMet.
As far as biomarkers are concerned, M25-287 mandates only that a patient's NSCLC cancer be EGFR-mutated. This trial's primary efficacy endpoints are PFS and ORR, so the baseline to beat is Tagrisso's registrational Flaura trial, where the AstraZeneca drug scored median PFS of 18.9 months, and ORR of 77% (median OS in Flaura was 38.6 months).
It's likely that AbbVie has higher hopes for teliso-A than it now has for Emrelis. The latter had consistently shown NSCLC activity mostly in patients with the highest levels of cMet overexpression.
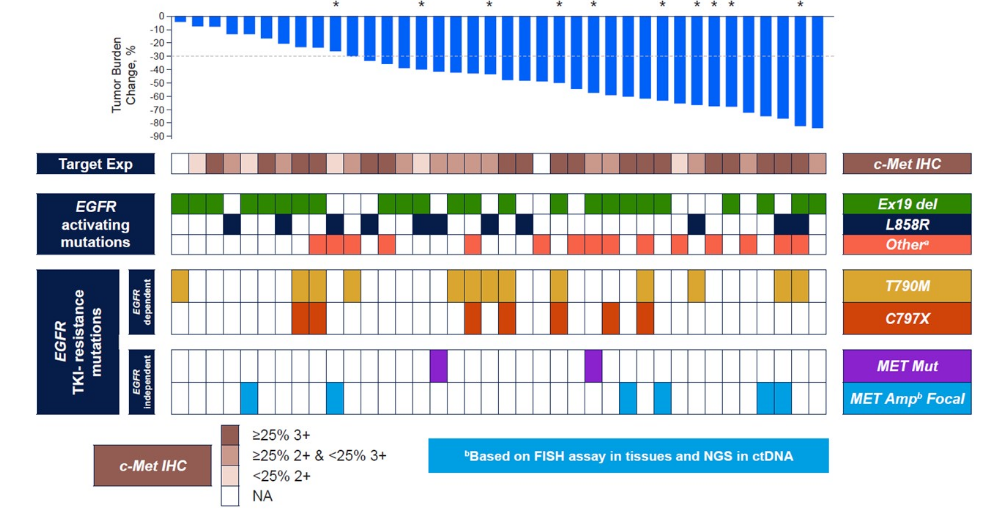
Some grounds for optimism were provided at the recent ASCO conference, which saw presentation of data from teliso-A's phase 1 basket trial focusing on a subgroup of 41 third-line or later EGFRm non-squamous NSCLC patients. Across all 41 patients a 63% ORR was seen, regardless of EGFR-activating and EGFR-TKI resistance mutations.
But perhaps even more impressive was activity in tumours with relatively low cMet expression. At <25% expression with IHC 3+ the ORR was still 60% (12 of 20 patients), while a more demanding analysis, <25% with IHC2+, produced two responses among six subjects.
Teliso-A in 3L+ EGFRm non-squamous NSCLC (basket study subgroup)
Another part of AbbVie's attack with teliso-A concerns cMet-overexpressing relapsed colorectal cancer, where first-in-human data at ASCO 2024 showed 2.4mg/kg or higher dose scoring a 38% ORR in a retrospective analysis of patients overexpressing cMet at ≥10% with IHC 3+, and a 14% ORR at cMet expression levels below 10%.
Teliso-A last year started the phase 3 Andrometa-CRC trial. This was known to enrol cMet overexpressers, but an ASCO 2025 trials-in-progress poster revealed the precise boundary: ≥10% with IHC 3+.
Key studies of AbbVie's cMet-targeting ADCs
| Trial | Setting | Design | Note |
|---|---|---|---|
| Emrelis (telisotuzumab vedotin) | |||
| Luminosity* | 2nd-line cMet+ve NSCLC | MonoRx, uncontrolled | Basis for US accelerated approval as Emrelis, in cMet ≥50% with IHC 3+ |
| Telimet NSCLC-01 | 2nd-line cMet+ve NSCLC | MonoRx, vs chemo | Confirmatory study |
| M22-142 | 2nd-line EGFRm cMet+ve NSCLC | Tagrisso combo, vs chemo | Withdrawn for “strategic considerations” |
| Telisotuzumab adizutecan | |||
| Andrometa-CRC | 3rd-line cMet+ve (≥10% with IHC 3+) colorectal cancer | MonoRx, vs Lonsurf + Avastin | Started Nov 2024 |
| M25-287 | 1st-line EGFRm NSCLC | Tagrisso combo, vs Tagrisso | cMet status must be available |
Note: *ph2, as all others are ph3 except M25-287, which is ph2/3. Source: OncologyPipeline.
2523