
ASH 2025 – Kelonia gets another response

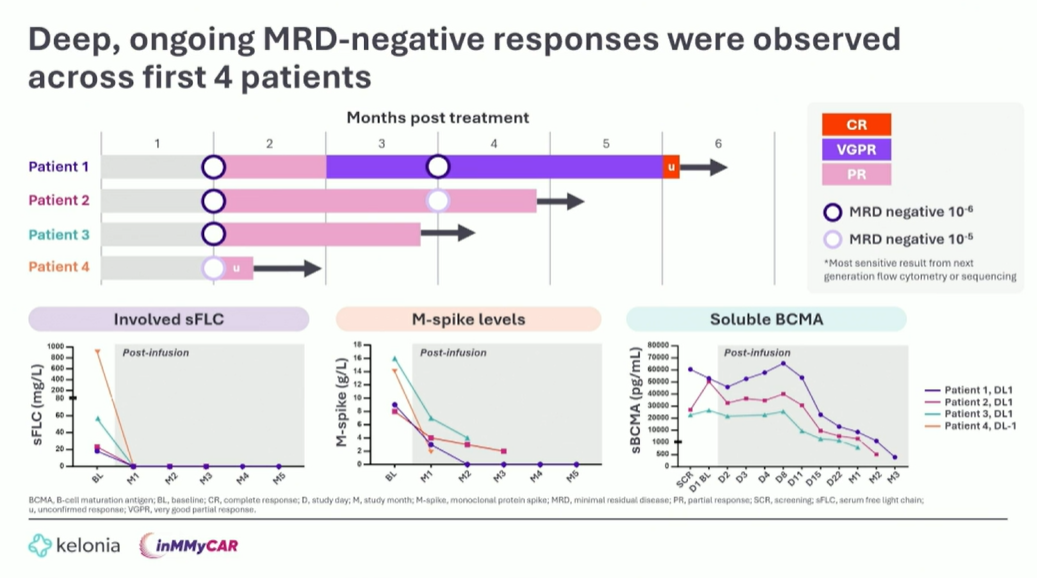
A late-breaking ASH abstract showed a 100% overall response rate in three patients receiving Kelonia’s BCMA-targeting in vivo Car-T KLN-1010 – and Tuesday’s presentation revealed another partial response. Although this is unconfirmed, it’s notable that the patient received a lower dose: 6x106IU/kg (DL -1) versus 2x107IU/kg (DL 1) for the first three patients. The decision to dose lower in the late-line multiple myeloma Inmmycar trial was down to “high levels of activity and a favourable tolerability profile” in the initial patients, said the presenter, Professor Joy Ho of Sydney’s Royal Prince Alfred Hospital. Although patient numbers are still small, there was no ICANS or delayed neurotoxicity, and all instances of cytokine release syndrome were grade 1-2, she noted. Cytopenia was also “minimal”, with only one case of grade 4 neutropenia, which resolved within 16 hours. The only other human data for in vivo Car-T therapy comprise case reports with AstraZeneca's Esobiotec-derived anti-BCMA project ESO-T01. KLN-1010's toxicity profile, along with the avoidance of lymphodepletion, could raise hopes that in vivo Car-T might eventually replace ex vivo autologous therapies, but Kelonia has a long way to go. The group plans to dose another two patients with DL -1, then decide on next steps, a spokesperson told ApexOnco.
2262













