
ASCO 2025 – BioNTech has a prostate decision to make

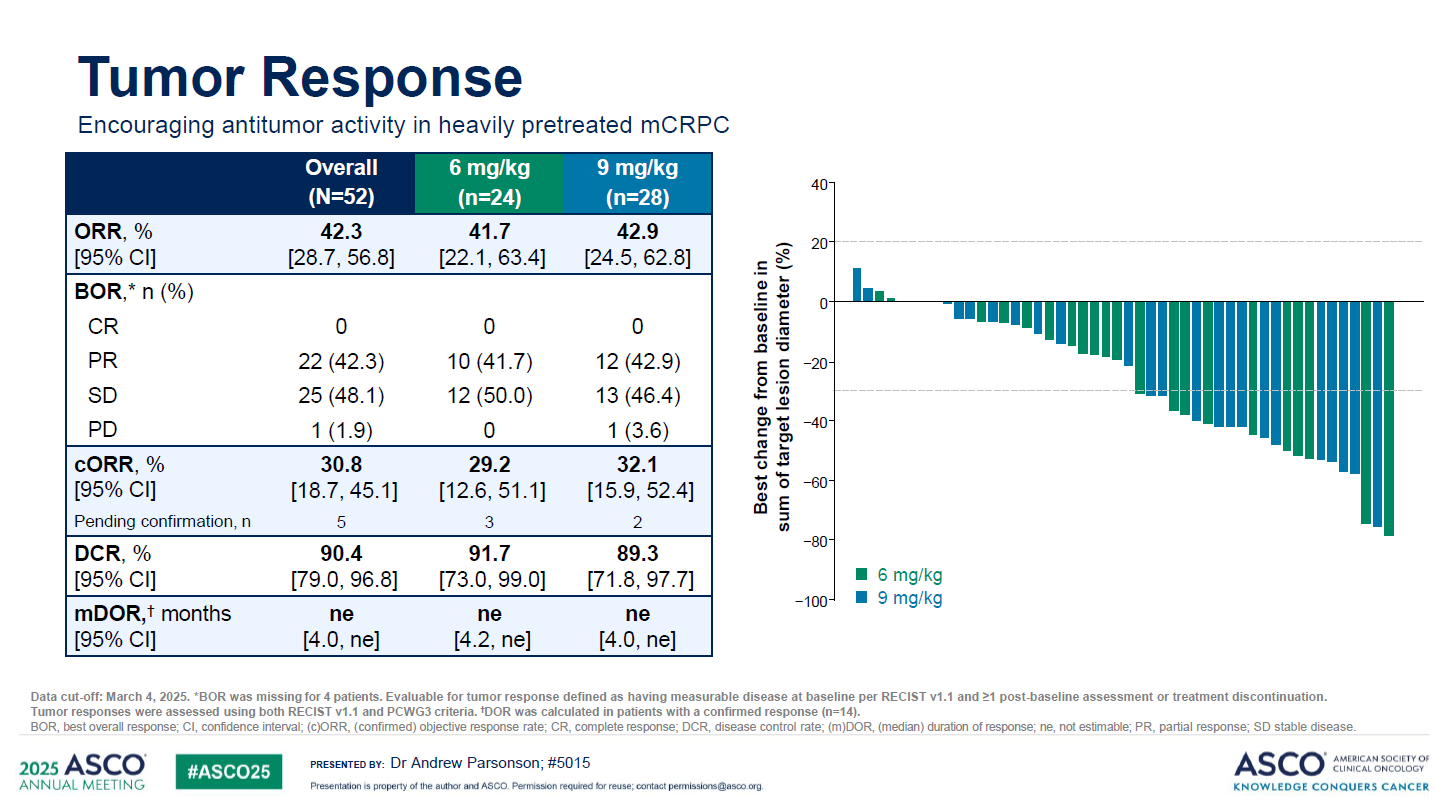
BioNTech’s DualityBio-originated B7-H3-targeting ADC BNT324 could have a new use, castrate-resistant prostate cancer, although the company is being cautious about taking this indication forward. Late-line mCRPC cohorts from a phase 1 solid tumour trial, reported at ASCO on Sunday, found a confirmed ORR of 31% across two doses – which looks favourable versus other early-stage prostate cancer assets in development, including J&J’s anti-KLK2 T-cell engager pasritamig, presented at the same session. However, BioNTech’s chief medical officer, Özlem Türeci, told ApexOnco the company hadn’t yet decided whether to push on with BNT324 in prostate cancer, saying: “We’re analysing other indications ... and then we’ll go forward.” One of those diseases is small-cell lung cancer, where BNT324 impressed at the ESMO Asia meeting last year. Other B7-H3 ADC contenders have already gone into phase 3 in SCLC, including Merck & Co/Daiichi’s ifinatamab deruxtecan, and GSK/Hansoh’s GSK5764227. And Merck recently started a pivotal trial of its project in mCRPC. Still, Türeci said BioNTech wouldn’t rush, and that the company was also looking at combining BNT324 with its PD-L1 x VEGF asset, BNT327; here a phase 1/2 combo lung cancer study recently begun, and a phase 2 is planned in other solid tumours.
3026













