
ASCO-GI – Astellas focuses on a degrader-chemo combo
A pivotal trial will test the KRAS G12D degrader setidegrasib plus chemo in first-line PDAC.
A pivotal trial will test the KRAS G12D degrader setidegrasib plus chemo in first-line PDAC.

Astellas hasn’t revealed much so far about the planned phase 3 trials of its KRAS G12D degrader setidegrasib, but a poster at the ASCO Gastrointestinal Cancers symposium has shed more light on the matter. A pivotal study in pancreatic ductal adenocarcinoma, slated to begin this year, will test an mFolfirinox chemo combo in first-line disease.
At ASCO-GI the group presented the first data with this combo from a phase 1 trial, claiming a 58% overall response rate in seven of 12 evaluable patients with front-line KRAS G12D-mutant disease. Two responses were unconfirmed, but even excluding these gives an ORR of 42%, which seems promising in a tough-to-treat cancer – although adverse events will be worth keeping an eye on.
Phase 1 data with setidegrasib + chemo in first-line PDAC
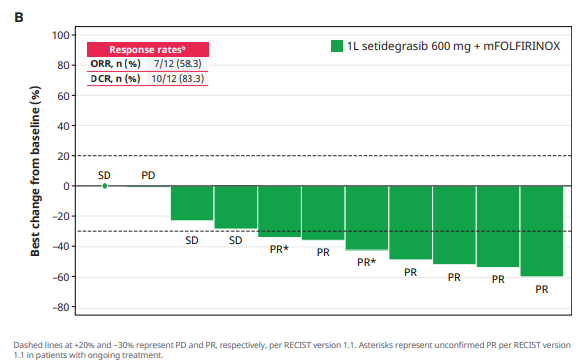
The latest efficacy results are in line with those seen with Revolution Medicines’ pan-RAS inhibitor daraxonrasib plus chemo. That regimen produced a confirmed ORR of 48% in the RMC-GI-102 study, although the populations involved were different, with Revolution’s study enrolling patients with various RAS mutations.
At the time Revolution looked like it could have a balancing act on its hands, with the combo doing slightly better than daraxonrasib monotherapy in terms of efficacy, but with a higher incidence of high-grade adverse events.
Astellas might be facing the same issue: setidegrasib 600mg plus chemo was linked with a 59% rate of grade 3 or higher adverse events, and 23% rates of both serious adverse events and drug-related discontinuations. There was a 32% rate of grade 3 or higher neutropenia but, on the plus side, no drug-related deaths.
And an Astellas spokesperson contended that the tolerability profile of setidegrasib plus mFolfirinox was "very similar" to that with mFolfirinox alone.
Monotherapy
Astellas also provided updated results on setidegrasib monotherapy in second and third-line PDAC, where the confirmed ORR was 24% among 21 patients receiving 600mg, the go-forward dose. Still, monotherapy doesn’t appear to be the focus now.
Overall, the results seem to justify Astellas’s perseverance with setidegrasib, previously known as ASP3082, which initially reported disappointing results at ESMO 2024 from the same phase 1 trial.
Phase 1 data with setidegrasib in second/third-line PDAC
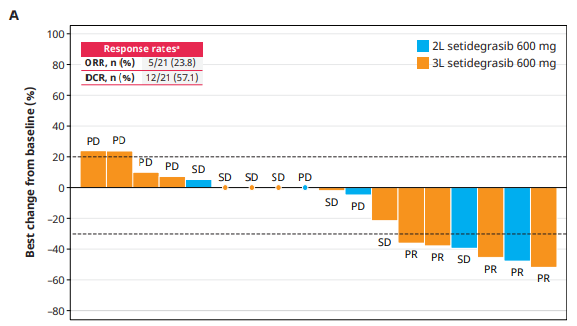
Astellas isn’t the only KRAS G12D player moving into pivotal development in pancreatic cancer. As well as Revolution’s planned Rasolute-303 front-line PDAC study of daraxonrasib, which had been expected to start last year, Jiangsu HengRui recently listed a Chinese pivotal study of its inhibitor, HRS-4642, while GenFleet is also beginning a second-line Chinese phase 3 of its Verastem-partnered asset, VS-7375 (GFH375).
But setidegrasib is the lead G12D degrader. Astellas has a back-up asset, ASP4396, in phase 1, that harnesses the cereblon E3 ligase for degradation; setidegrasib uses von Hippel-Lindau (VHL) E3 ligase.
Meanwhile, Arvinas took its degrader, ARV-806, into the clinic last year, with data expected in 2026. Another contender is Ranok Therapeutics’ RNK08954, in phase 2 in China.
Setidegrasib also showed promise last year in relapsed NSCLC, and pivotal trials in this cancer are in the planning stages, although again the company hasn’t given details. Astellas also sees potential in colorectal cancer, and will make a decision soon on whether to push forward here.
2017













