
Epkinly gets a second-line follicular nod
And AbbVie and Genmab scoop their ASH presentation with new data.
And AbbVie and Genmab scoop their ASH presentation with new data.

AbbVie and Genmab have got one up on their anti-CD20 T-cell engager rivals with FDA approval for Epkinly in second-line follicular lymphoma. The partners’ closest competitor, Roche, isn’t due to report data until next year with its similarly acting therapy Lunsumio.
The US nod, for Epkinly plus Rituxan and Revlimid, has also seen the release of updated numbers from the supporting Epcore FL-1 trial, ahead of that study’s presentation at the ASH meeting in December.
As toplined in August, Epcore FL-1 met its primary endpoint, with the Epkinly/Rituxan/Revlimid combo producing a 79% reduction in the risk of progression or death versus Rituxan plus Revlimid.
Now AbbVie and Genmab have disclosed that median progression-free survival wasn’t reached with the triplet, compared with 11.2 months for the doublet. The updated label also includes survival curves, which until now hadn't been expected until ASH.
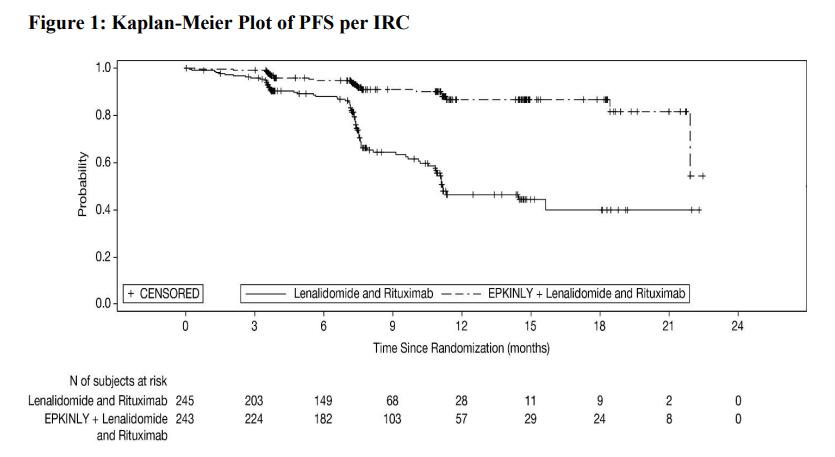
Previously, the companies disclosed an ORR of 96% with the triplet; this has now come down to 89%, but this was still statistically significantly higher than the 74% seen with the doublet, with a p value of less than 0.0001.
Complete response rates were 74% with the triplet and 43% with the doublet.
As for safety, the partners disclosed one case of immune effector cell-associated neurotoxicity syndrome (ICANS), at grade 1. The ASH abstract notes a higher rate of grade 3/4 adverse events with the triplet versus the doublet (88% vs 62%), primarily driven by higher rates of grade 3/4 neutropenia (66% vs 38%) and infections (29% vs 13%). As of a 10 January 2025 cutoff date, there were no fatal adverse events deemed related to Epkinly, according to the abstract.
Epcore FL-1 is set to be presented at ASH on Sunday 7 December, and will be featured in the meeting's press programme.
Accelerated to full
Epkinly, which is given subcutaneously, gained accelerated approval in June 2024 as monotherapy for third-line plus follicular lymphoma. The latest FDA thumbs up also converts this into a full approval.
Roche’s Lunsumio, which is given intravenously, got an accelerated nod in third-line follicular in December 2022, but has since fallen behind. Readout of that drug’s second-line pivotal study, Celestimo, was recently pushed back from 2025 to 2026; it had once been expected to yield data in 2023.
Roche is taking a different approach from AbbVie and Genmab: rather than opting for a triplet, the Swiss group is testing Lunsumio plus Revlimid, versus Rituxan plus Revlimid.
Regeneron is taking a similar approach with its contender odronextamab, in the second-line Olympia-5 trial; however, that project is further behind, having received two CRLs in follicular lymphoma, the latest on manufacturing issues. That drug has been approved in the EU as Ordspono since August 2024, both in third-line follicular lymphoma and DLBCL.
Roche had some better news this week with conditional approval of subcutaneous Lunsumio in the EU for relapsed/refractory follicular lymphoma. The SC formulation is awaiting an FDA decision, which had once been expected by 22 September, but was delayed by three months following an amendment to the application, “which included additional data required as part of the filing”, a spokesperson told ApexOnco.
All three assets are also in first-line follicular lymphoma studies, but results look some way off.
Anti-CD20 T-cell engagers in follicular lymphoma
| Product | Company | 3rd-line | 2nd-line | 1st-line |
|---|---|---|---|---|
| Epkinly | AbbVie/ Genmab | AA Jun 2024 for monoRx (Epcore NHL-1 trial); converted to full approval Nov 2025 | Full approval Nov 2025 (Rituxan + Revlimid combo, Epcore FL-1 trial) | Epcore FL-2 (Rituxan + Revlimid combo), data due 2030 |
| Lunsumio | Roche | AA Dec 2022 for monoRx (GO29781 trial) | Celestimo (Revlimid combo), data due 2026 (previously 2025) | MorningLyte* (Revlimid combo), completes Nov 2028 |
| Ordspono | Regeneron | 2 CRLs, latest Aug 2025 on manufacturing issues | Olympia-5 (Revlimid combo), completes Jan 2029 | Olympia-1 (monoRx), completes Feb 2028; Olympia-2 (chemo combo), completes Jul 2029 |
Note: *investigator-sponsored trial. Source: OncologyPipeline.
946













