
ESMO 2025 – Merck and Daiichi Rejoice over raludotatug

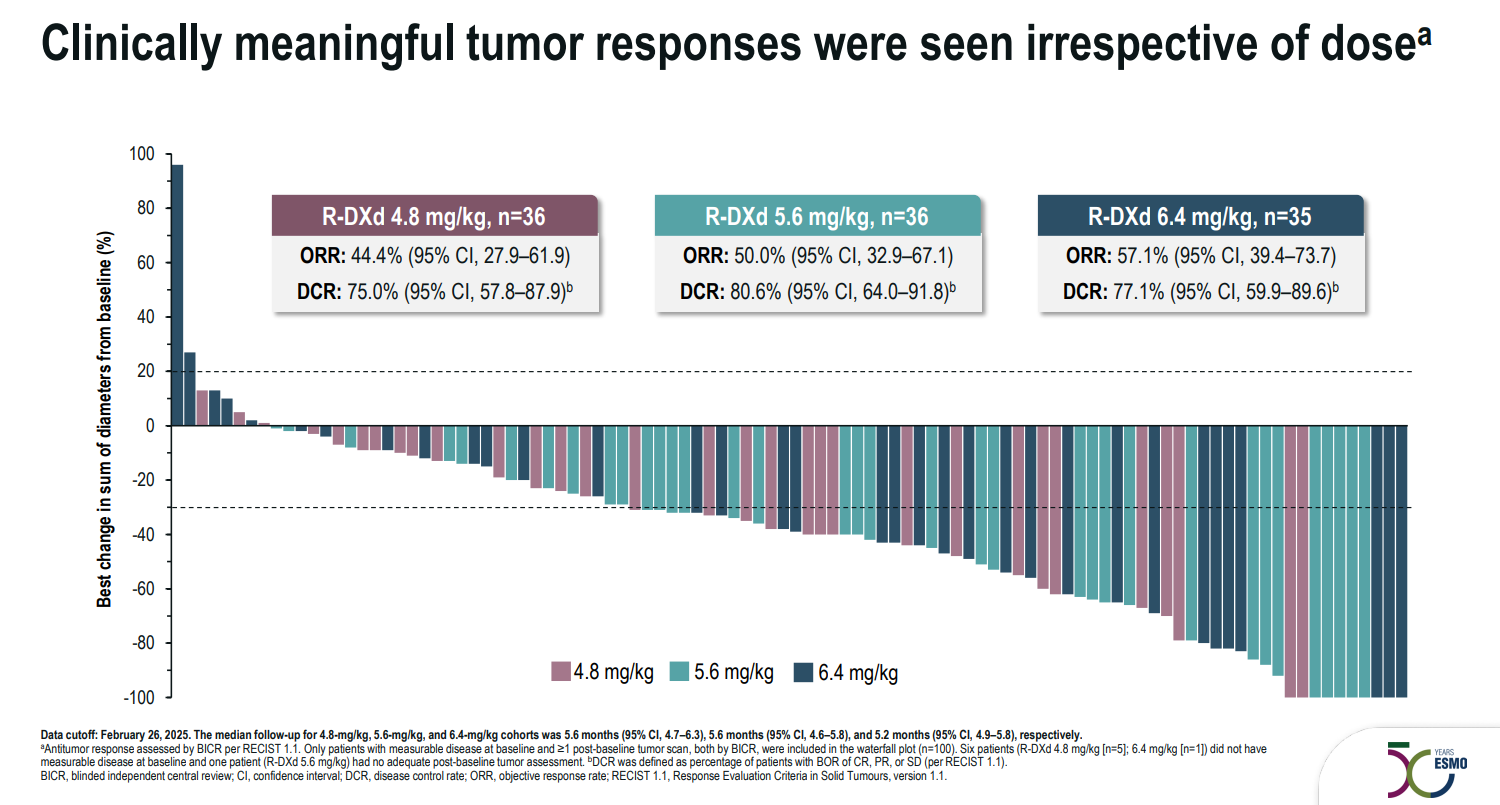
Following problems with one Daiichi-originated ADC, patritumab deruxtecan, Merck & Co has had more cheerful news with another project involved in its monster deal, the CHD6-targeting raludotatug deruxtecan. Data presented at ESMO on Sunday from the phase 2 portion of the phase 2/3 Rejoice-Ovarian01 trial showed impressive response rates in platinum-resistant ovarian cancer, exceeding a 46% confirmed ORR seen in an earlier phase 1 trial. Across three doses tested in Rejoice-Ovarian01, 4.8mg/kg, 5.6mg/kg and 6.4mg/kg, confirmed ORR was 51% among 107 patients. The investigators concluded that 5.6mg/kg, with a 50% ORR, provided the best risk/benefit profile; however, there could still be reasons to be cautious. Any-grade treatment-related interstitial lung disease – a known toxicity with deruxtecan payloads – was seen in two patients at 6.4mg/kg, but one grade 3 case was seen with 4.8mg/kg, suggesting that lower doses aren’t immune to this issue. Data on progression-free survival and duration of response will be crucial, but aren’t yet mature. The phase 3 portion of Rejoice-Ovarian01 will evaluate ralu-dxd at 5.6mg/kg versus physician’s choice of chemo; ORR and PFS are co-primary endpoints. Merck is also awaiting an FDA decision on Keytruda plus chemo in platinum-resistant ovarian cancer, following a win in Keynote-B96.
3814













