
ESMO 2025 – iza-bren’s US backing comes with toxicity

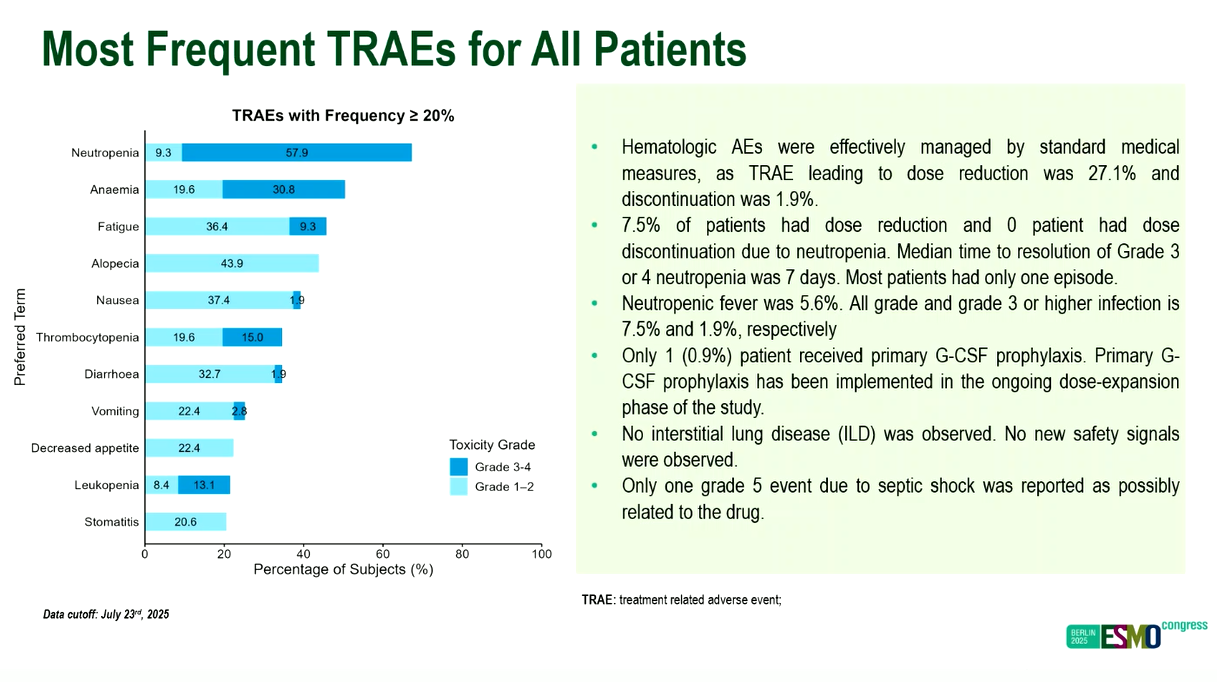
Barely a month after SystImmune revealed Chinese data on izalontamab brengitecan that backed Bristol Myers Squibb's move into pivotal trials, the results have been mirrored in a US study presented at ESMO on Friday. However, the latest numbers have been tinged with the revelation that G-CSF prophylaxis has been mandated to mitigate against haematological side effects in the phase 1 study, after grade 3/4 neutropenia was seen in an astonishing 58% of patients, a fact the discussant, Dr Fiona Thistlethwaite, called "not insignificant in this population". Bristol stated that “haematologic adverse events were effectively managed by standard medical measures”. The trial, described as iza-bren’s first global study to yield data, mostly concerned lung cancer, plus several other heavily pretreated tumours, and among 63 patients yielded a confirmed response rate of 35%. This comprised 1.5-3.0mg/kg iza-bren doses, of which the most impressive was 2.5mg/kg, which gave a 55% ORR in 20 subjects. This appeared to replicate data in September from a Chinese trial, in which SystImmune zeroed in on 50 post-TKI, chemo-naive NSCLC patients given 2.5mg/kg, and reported a 56% ORR. Bristol paid SystImmune $800m to license iza-bren, and is now running three pivotal studies of the anti-EGFR x HER3 ADC.
Adverse events in phase 1 US study of izalontamab brengitecan
2347













