
ESMO 2025 – Merck shoots for an ovarian cancer first

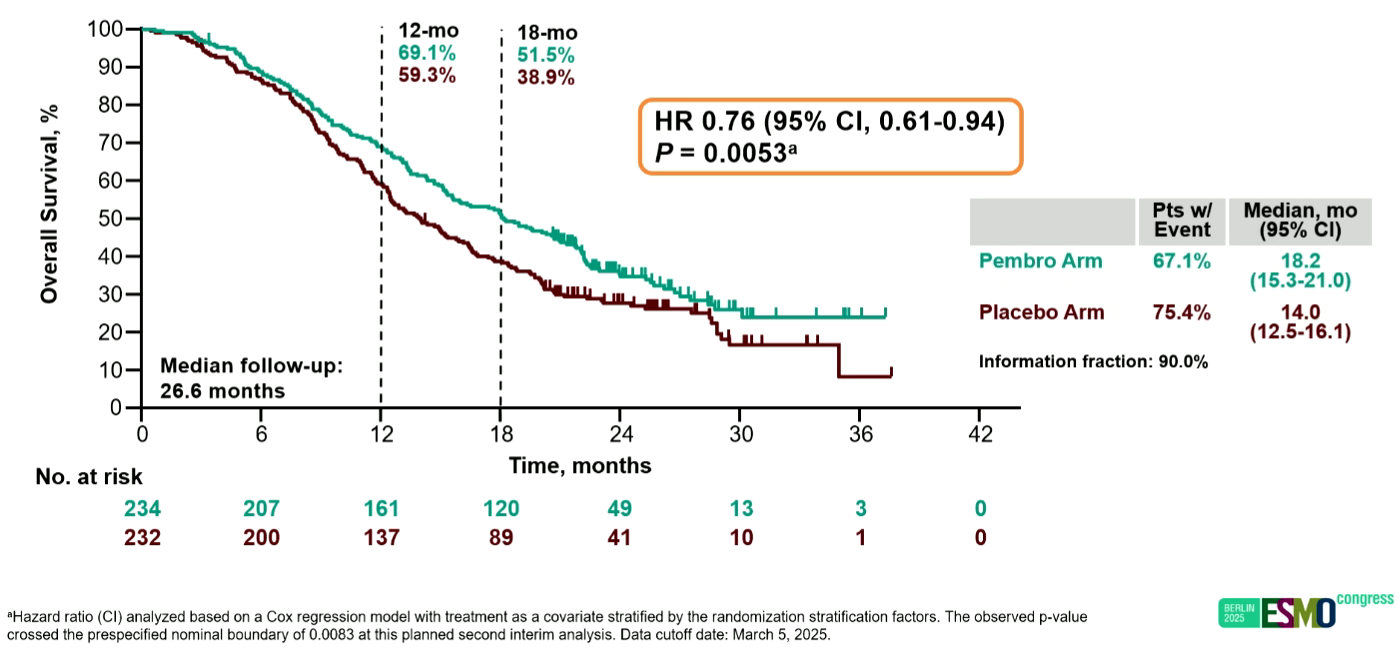
Platinum-resistant ovarian cancer is one of few settings into which anti-PD-(L)1 therapies haven’t broken, but this might be about to change. That’s courtesy of Merck & Co’s Keynote-B96 study, testing Keytruda/chemo against chemo alone, toplined as positive in May and presented at an ESMO presidential session this weekend. At the time of the May release Keynote-B96’s key secondary overall survival endpoint had succeeded only for PD-L1 expressers, and this was the state of play as of the 5 March data cutoff to which the ESMO presentation related. But this is already out of date, Merck having announced last Thursday that at final analysis Keynote-B96 yielded a “statistically significant and clinically meaningful” OS improvement in all-comers too. Keynote-B96 had a hierarchical statistical design, and for the record its PFS endpoints (primary in PD-L1 ≥1%, and secondary in all-comers) were both hit at first interim. The OS hit in PD-L1 ≥1% expressers, who made up 73% of Keynote-B96’s population, came at second interim, and amounted to a 24% reduction in risk of death (p=0.0053). No data have yet been revealed about the final OS all-comers success, but Merck has filed with the FDA, which has set a 20 Feb 2026 PDUFA date.
Keynote-B96 overall survival in PD-L1 ≥1% expressers at second interim analysis
3078













