
ESMO 2025 – Roche sticks it to its SERD rivals
But it will be up to regulators to decide if giredestrant has done enough for an all-comers label.
But it will be up to regulators to decide if giredestrant has done enough for an all-comers label.

When Roche toplined a positive result with giredestrant in all comers, in the Evera trial in second-line ER-positive, HER2-negative breast cancer, the big question was how this oral SERD had performed in patients without ESR1 mutations. The answer came at ESMO on Saturday, when full Evera data showed giredestrant to have done better than most other oral SERDs in the ESR1 wild-type population.
Whether this is down to giredestrant, smart trial design or the addition of everolimus to the regimen tested is the next question. That's not to say that there weren't some promising signs in patients without ESR1 mutations, and Roche has at least managed to do what Lilly and Arvinas/Pfizer couldn't. Now it’s down to the FDA to decide whether this is enough for an all-comers label.
One potential explanation for the all-comers win is that Roche was clever in designing Evera, by enriching the trial for ESR1m patients. Numbers released at ESMO show that around 55% of patients in the study had ESR1m, versus the roughly 40% of patients in the general population who develop ESR1m following endocrine therapy.
However, other factors could also be in play. In Evera giredestrant was combined with everolimus, and compared against endocrine therapy plus everolimus. Discussing the results, Dr Alessandra Gennari of University of Piemonte Orientale, Italy, suggested that the combination might have been a reason for the positive all-comers result.
Even so, when opining on where giredestrant might fit into the treatment paradigm, she still highlighted ESR1m as the population of choice.
All-comers benefit
Evera had co-primary endpoints of investigator-assessed progression-free survival in the intent-to-treat population, as well as in ESR1m disease. Overall survival was a secondary endpoint.
At ESMO Roche reported that in the ITT population median PFS was 8.8 months with giredestrant plus everolimus, versus 5.5 months with control, equating to a 44% reduction in the risk of progression or death.
This was driven by the ESR1m group, where mPFS was 10.0 months versus 5.5 months, leading to a 62% decrease in the risk of progression or death.
There was also a trend towards an OS benefit in both groups, with a hazard ratio of 0.69 in all comers, and 0.62 in ESR1m.
But even in ESR1 negatives, an exploratory analysis showed some benefit on PFS; though medians were virtually identical, and 5.7 months with giredestrant plus everolimus versus 5.5 months with control, across the whole trial the hazard ratio was a more impressive seeming 0.84. Even though the median numbers weren’t that different, survival curves do show separation.
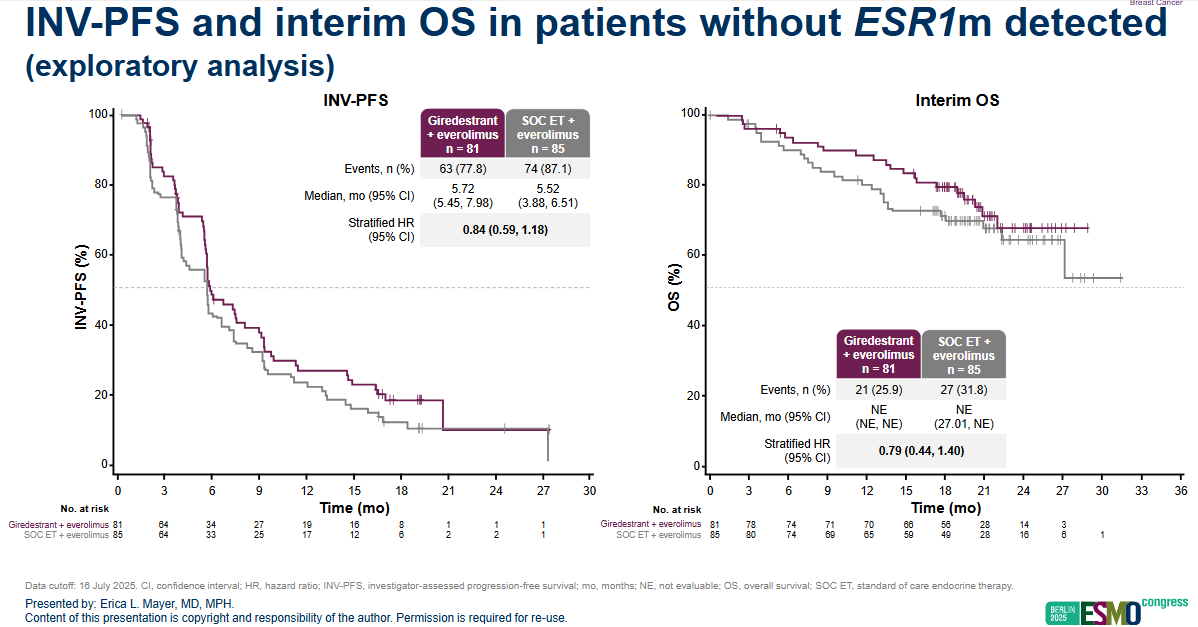
The only currently FDA approved oral SERD is Menarini’s Orserdu, which is indicated for ESR1m disease only, despite showing a benefit in an all-comers population in its pivotal study, Emerald. An exploratory analysis of PFS in patients without ESR1 mutations showed a hazard ratio of 0.86 – similar to the HR in Evera, which could be a bad sign for Roche. Around 50% of patients in Emerald had ESR1 mutations.
Lilly's imlunestrant, now branded Inluriyo, was FDA approved in the second line last month based on the Ember-3 trial, but only in ESR1m – although a combination with Verzenio did show a benefit in all comers, suggesting a broader path forward for that agent.
Meanwhile, Arvinas and Pfizer have turned away from their project, vepdegestrant, and are trying to find a partner to take it forward, after showing a second-line benefit only in ESR1m patients.
Meanwhile, AstraZeneca has said that second-line disease is no longer a focus for its oral SERD, camizestrant.
Cross-trial comparison of oral oestrogen degraders in second-line ER-positive HER2-negative breast cancer
| Project | Company/ies | Study | Control | Primary endpoint(s) | Result |
|---|---|---|---|---|---|
| Giredestrant (+ everolimus) | Roche | Evera* | Investigator's choice endocrine therapy + everolimus | PFS in all-comers | 8.8 vs 5.5 mths; HR=0.56; p<0.0001 |
| PFS in ESR1 mutant | 10.0 vs 5.5 mths; HR=0.38; p<0.0001 | ||||
| PFS in ESR1 wild-type | 5.7 vs 5.5 mths; HR=0.84 | ||||
| Orserdu (elacestrant) | Menarini | Emerald* | Investigator’s choice | PFS in all-comers | 2.8 vs 1.9 mths; HR=0.70; p=0.0018 |
| PFS in ESR1 mutant | 3.8 vs 1.9 mths; HR=0.55; p=0.0005 | ||||
| Imlunestrant | Lilly | Ember-3** | Exemestane or Faslodex | PFS in all-comers | 5.6 vs 5.5 mths; HR=0.87; p=0.12 |
| PFS in ESR1 mutant | 5.5 vs 3.8 mths; HR=0.62; p<0.001 | ||||
| Vepdegestrant | Arvinas/Pfizer | Veritac-2 | Faslodex | PFS in all-comers | 3.7 vs 3.6 mths; HR=0.83; p=0.07 |
| PFS in ESR1 mutant | 5.0 vs 2.1 mths; HR=0.57; p=0.0001 |
Notes: *trial enriched for ESR1m disease; **also includes imlunestrant + Verzenio arm. Source: ESMO & OncologyPipeline.
This story has been updated.
3204













