Incyte does TGF-beta differently
Four months after yielding first-in-human data INCA33890 enters phase 3.
Four months after yielding first-in-human data INCA33890 enters phase 3.

The high-profile failure of Merck KGaA/GSK’s bintrafusp alfa dealt a severe blow to the idea that inhibiting TGF-β could boost the efficacy of PD-(L)1 blockade, but Incyte is undeterred. And so confident is the company that this approach still has legs that it has just revealed a phase 3 study of its contender, INCA33890.
The trial is to begin next month, according to a new listing on clinicaltrials.gov, and the move is all the more extraordinary because it’s being taken on the back of no confirmatory data beyond a small first-in-human trial, which yielded its first results at ESMO last October. Incyte accepts the poor precedent of this approach, but reckons its molecule is different.
The theory is that TGF-β signalling can be a driver of resistance to immune checkpoint blockade by stopping immune system cell tumour infiltration in the tumour microenvironment. Bintrafusp alfa was an anti-PD-L1 x TGF-β “trap” fusion protein, and in 2019 GSK paid Merck KGaA €300m up front to develop the project jointly.
But the deal was terminated after bintrafusp failed to beat Keytruda in first-line lung cancer; other disappointments include BioNTech’s Biotheus-derived anti-PD-L1 x TGF-β bispecific PM8001 (no longer listed in the company’s pipeline, though formally still under review), and the monospecifics nisevokitug, an anti-TGF-β1/2 MAb discontinued by Novartis, and the TGF-βR1 inhibitor PF-06952229, canned by Pfizer.
Conditionally active
Incyte’s ESMO presentation specifically called out such approaches, saying that broad TGF-β pathway inhibitors, including TGF-β/PD-(L)1 trap molecules and TGF-β small-molecule inhibitors, had shown limited efficacy and/or excess toxicity.
With this in mind, INCA33890 was designed as an anti–TGF-βR2 x PD-1 bispecific common light-chain MAb that aims specifically to target immune cells in the tumour microenvironment. This is achieved through conditional activation: INCA33890 blocks the TGF-βR2 receptor only when it’s also bound to PD-1, meaning that in PD-1-negative non-target cells it doesn’t inhibit TGF-β signalling.
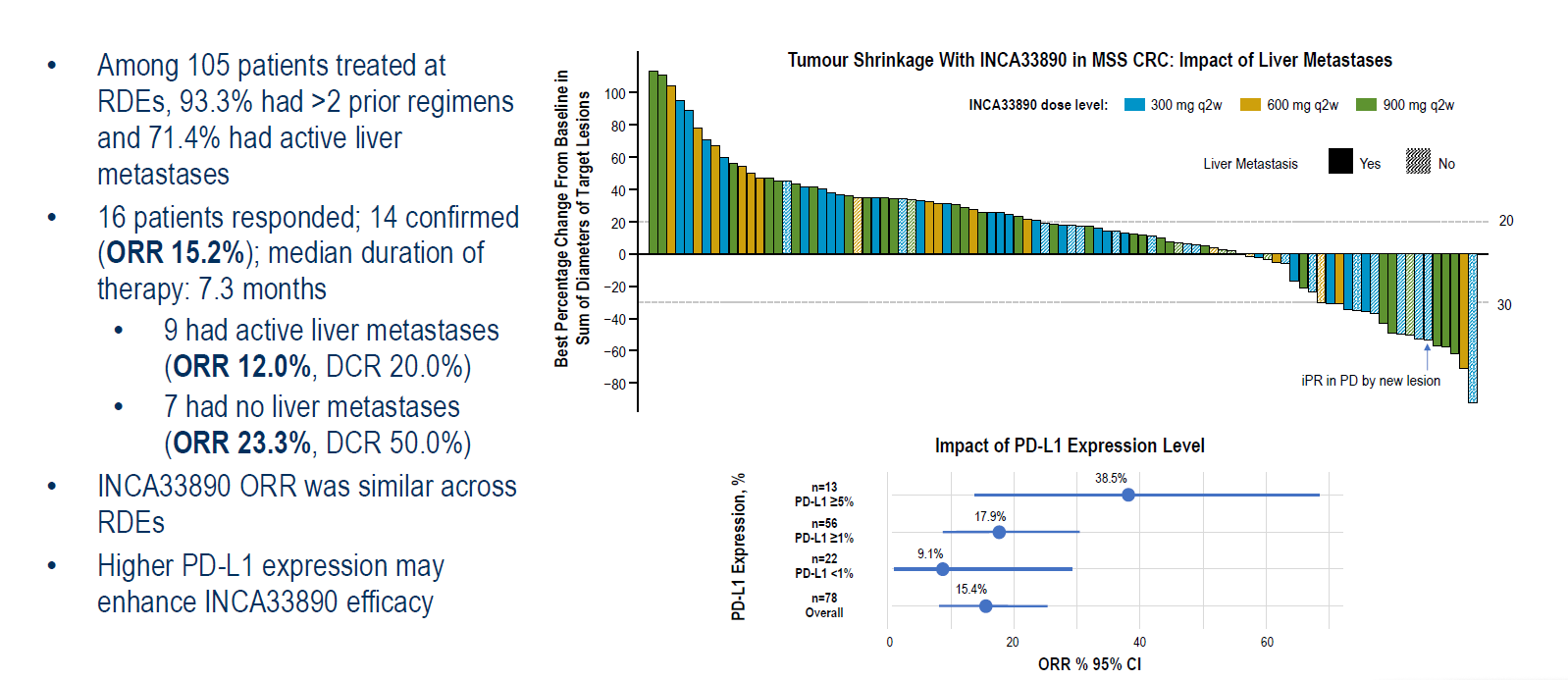
The only human trial Incyte has run to demonstrate whether this holds true is a phase 1 in 408 patients with various tumour types. This yielded its first data at ESMO, the highlight of which was a 13% confirmed response rate among 105 patients with microsatellite-stable (MSS) colorectal cancer given doses of 300-900mg.
13% might on the face of it seem underwhelming, but MSS colorectal cancer is an especially intractable tumour type, which can’t be treated with PD-(L)1 blockade, and over 90% of the patients were at least third line. There were also indications that INCA33890 worked best in cancers with at least 5% PD-L1 expression.
Phase 1 data for INCA33890 in MSS colorectal cancer
Still, despite its conditionally acting mechanism, INCA33890 was associated with immune-related adverse events in 31% of patients. INCA33890 up to 1.2g was said to be well tolerated, but 1.5g exceeded the maximum tolerated dose, yielding myocarditis, diabetes-related ketoacidosis, encephalomyelitis and adrenal insufficiency.
900mg every two weeks was deemed the recommended phase 2 dose, so presumably this is what will be tested in the just disclosed phase 3 trial, which is due to start on 16 February.
This will test INCA33890 plus Avastin and Folfox chemo, against Avastin/Folfox alone, in 700 patients with first-line MSS colorectal cancer, and test progression-free survival as sole primary endpoint. The first-line setting obviously gives Incyte a chance to show better activity than INCA33890 did in phase 1, but the phase 3 study sets no lower limit on PD-L1 expression.
1950













