
Ipsen sees a future in gammadelta T-cells
The company will acquire ImCheck for €350m.
The company will acquire ImCheck for €350m.

Ipsen is expanding its immuno-oncology pipeline with the acquisition of ImCheck Therapeutics, in a deal worth €350m up front. This represents a meaningful bet on the promise of gammadelta T cells, an approach that has yet to meet the promise that it was once said to have.
The deal gives Ipsen control of ICT01, ImCheck’s lead candidate, a monoclonal antibody. ICT01 binds to BTN3A, a cell-surface protein also known as CD277, altering its conformation and leading to the activation of V9γδ2 T cells. Future milestones give the takeover, announced on Wednesday, a biodollar value of €1bn.
Gammadelta T-cells represent around 5% of the total T-cell population, and have demonstrated antitumor activity in preclinical studies, being said to be less susceptible to inhibition by immune checkpoints. This biology has attracted interest from multiple companies seeking to exploit γδ T-cell recruitment and activation for cancer therapy.
Still, the field has seen its share of setbacks. Earlier this year, Lava Therapeutics, once one of the frontrunners in the space, discontinued its lead asset and was bought by Xoma. This was in spite of Lava having attracted no less than Johnson & Johnson and Seagen as partners for its γδ T-cell engager projects.
In8bio has also worked on γδ T-cell engagement, while OncologyPipeline reveals Biosion and Shattuck as the only other companies also working specifically on BTN3A/CD277, both preclinically.
Early data
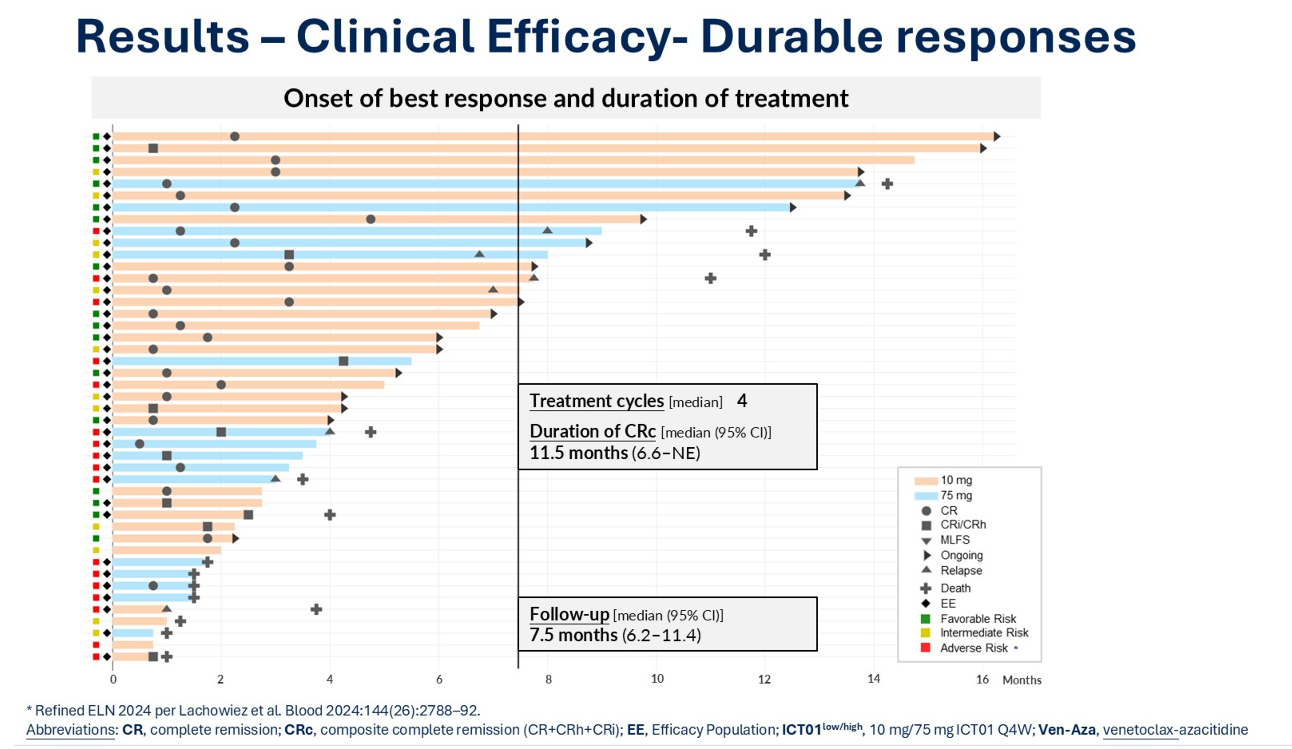
For ImCheck, the acquisition comes on the heels of positive early data for ICT01. At ASCO in May the company presented results from Eviction, a phase 1/2 study that evaluated the antibody in combination with Vidaza and Venclexta in newly diagnosed acute myeloid leukaemia patients.
At the 10mg dose the combo achieved 96% CRc and 74% complete response rates in the intent-to-treat population, and in patients with TP53-mutated AML the numbers were 83% for CRc and 60% for CR. At a median follow up of 8.5 months, the nine-month overall survival rate reached 83%. Grade 3/4 febrile neutropenia was observed in 42% of patients, and neutropenia in 71%.
Ipsen says it plans to kick off a phase 2/3 study next year.
ICT01 data from the phase 1/2 Eviction trial
2052













