
Ivonescimab back in tune
China's Harmoni-A trial hits on overall survival, but hopes for a global phase 3 seem optimistic.
China's Harmoni-A trial hits on overall survival, but hopes for a global phase 3 seem optimistic.

Summit and Akeso are facing questions about their anti-PD-1 x VEGF project ivonescimab, but the companies got a boost on Tuesday with the quiet revelation of an overall survival win in the Chinese Harmoni-A study in second-line lung cancer.
The result, which Akeso slipped into its six-month report, could boost hopes for the global Harmoni trial, which yielded disappointing OS data on the eve of ASCO. Still, a lack of details from Harmoni-A, and the manner in which the win was disclosed, might have done little to convince the sceptics.
Indeed, Summit’s stock, which has been on a rollercoaster ride this year, closed up 3% on Tuesday, but closed down 8% on Wednesday, perhaps as the low-key update filtered through.
Harmonising
Harmoni-A tests ivonescimab plus chemo, versus chemo alone, in Chinese EGFR-mutant NSCLC patients who had progressed after EGFR TKI therapy.
Harmoni has enrolled an analogous population, including US and other western sites, but also incorporates a large proportion of patients from Harmoni-A, with these making up around two thirds of Harmoni's population. This makes the latest disclosure highly relevant to the global trial.
At ASCO 2024 Harmoni-A showed a highly statistically significant benefit on PFS, with a hazard ratio of 0.46. This was enough to get ivonescimab Chinese approval.
At the time OS data were immature, but were showing worrying signs. The problem was that the result here appeared to be deteriorating: at 32% data maturity the study showed a 28% reduction in risk of death, but this shrunk to 20% at 52% data maturity.
Harmoni-A OS data
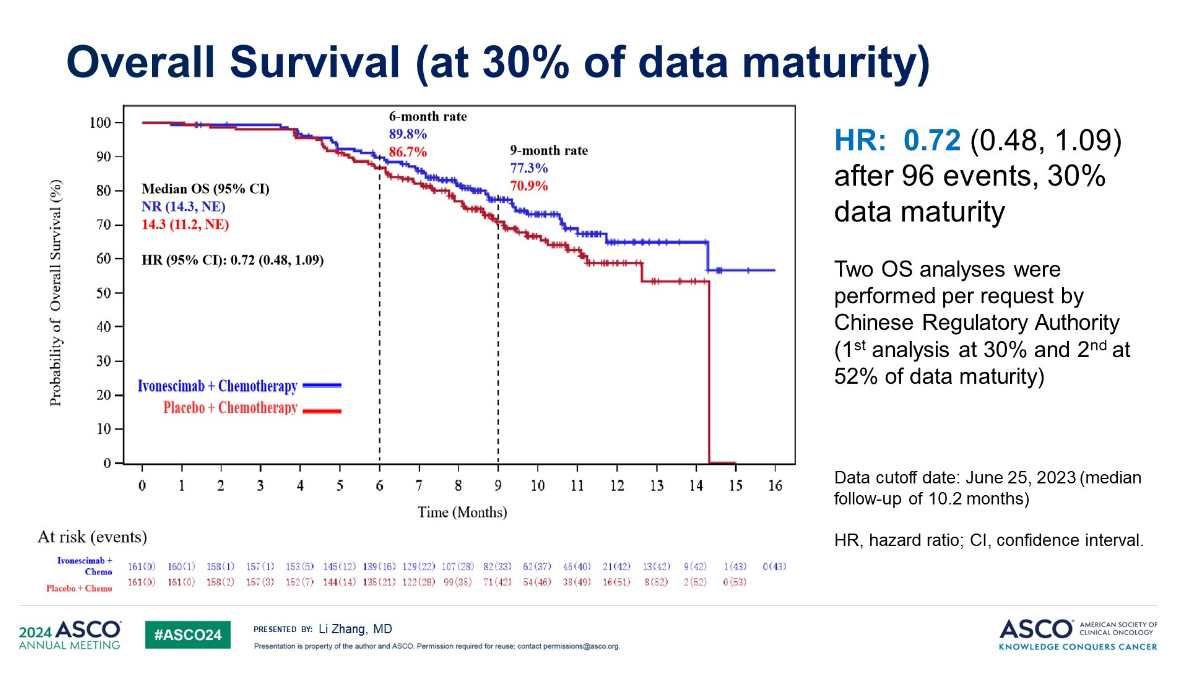
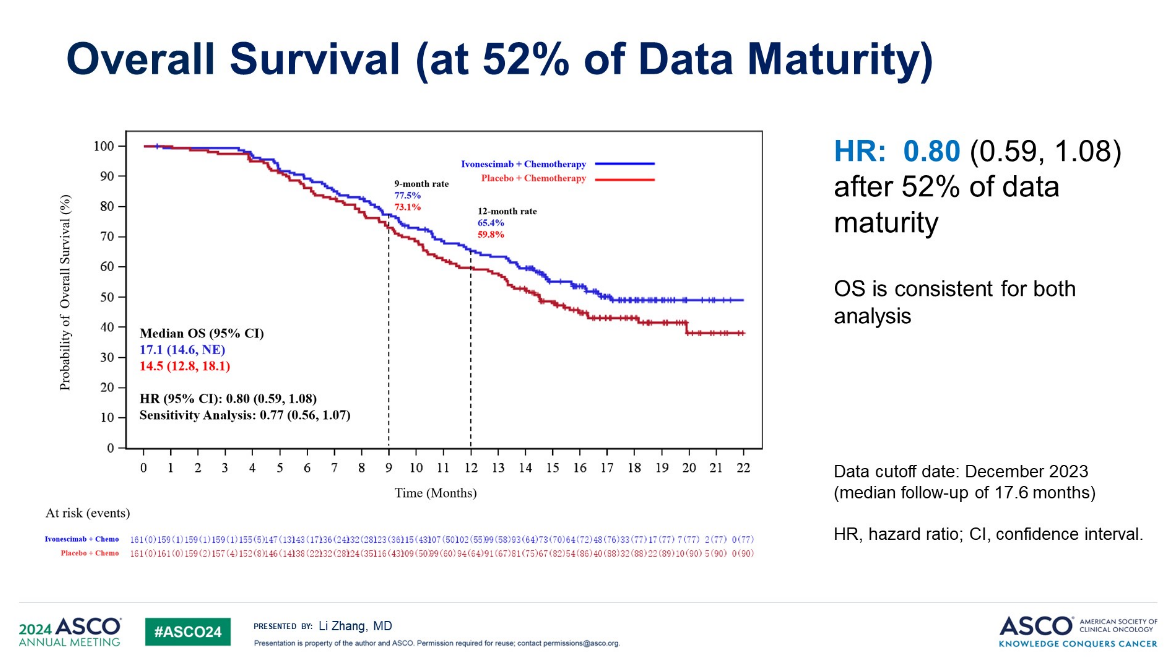
Akeso’s latest disclosure, which was brought to widespread attention by Evercore ISI’s Cory Kasimov, is therefore a surprise. All Akeso said was that in August 2025 the final OS analysis of Harmoni-A showed a “statistically significant and clinically meaningful OS benefit” with ivonescimab plus chemo. The company confirmed to ApexOnco that this was a new disclosure.
Detailed results of this study will be presented at an upcoming medical conference; the ESMO meeting in October is a potential venue.
However, a key question is how meaningful this result will be, given the previous deterioration in hazard ratios, and the possibility that this might have worsened further.
World Lung
Before that, all eyes will be on the global Harmoni trial, detailed data from which will feature at the World Lung conference in September.
Summit released topline Harmoni results in May, but only gave hazard ratios and p values. Although the PFS hazard ratio looked good the Chinese contingent appeared to have outperformed ex-China patients.
But the bigger worry is OS: the FDA has said that a regulatory submission can't be made without a statistically significant OS result, and here ivonescimab fell short, with the topline analysis showing a hazard ratio of 0.79 (p=0.057).
Kasimov speculated that the latest disclosure from Harmoni-A might increase the chance of Harmoni hitting on OS with further follow up. However, the fact the Harmoni OS p value has already been disclosed as above 0.05 is a mark against this optimistic read. It’s unclear what Harmoni data might be presented at World Lung, with only the title so far having been disclosed.
Ivonescimab has spurred a raft of copycats and increasingly rich deals by big pharma clamouring to get into this space. The latest disclosure seems like a positive step, but Summit still has to show that the Chinese data generated by Akeso aren’t a fluke.
3341













