
ASCO 2024 – Summit peaks on ivonescimab surprise
The PD-1 x VEGF bispecific apparently bests Keytruda, but questions remain.
The PD-1 x VEGF bispecific apparently bests Keytruda, but questions remain.

Summit Therapeutics is already a surprise winner of the ASCO weekend, but the results that sent its stock up 272% yesterday will not be presented at ASCO. Instead, the group’s boost came from Harmoni-2, an Akeso-sponsored Chinese head-to-head study of ivonescimab, a PD-1 x VEGF bispecific, against Keytruda in first-line NSCLC, which Summit toplined as positive yesterday.
Meanwhile, ASCO saw data today on ivonescimab from the Chinese Harmoni-A chemo combo trial in EGFR-mutant NSCLC cancer patients who had progressed on an EGFR TKI like Tagrisso. There are still plenty of questions for the company, including whether the Harmoni-2 data in particular can be replicated in western trials, but for now Summit’s $500m licensing of ivonescimab from China’s Akeso looks like a canny move.
Better than Keytruda?
PD-1 and VEGF inhibitors are already used in NSCLC, but Summit has long claimed that ivonescimab could have improved efficacy over using two separate agents against these targets.
Yesterday the group said that ivonescimab had "decisively" beaten Keytruda in Harmoni-2, marking the first time a phase 3 trial project had bested Keytruda in a head-to-head study in NSCLC.
However, all the group disclosed was that the study, which enrolled patients with PD-L1 expression ≥1%, met its primary endpoint of PFS. Summit described the PFS benefit as “clinically meaningful”, adding that this was seen across various subgroups, including patients with low and high PD-L1 expression, and squamous and non-squamous histologies.
However, as well as questions about the replicability of Chinese data, PFS has not always been a reliable predictor of overall survival in immuno-oncology.
In addition, perhaps it’s not that surprising that a PD-1 x VEGF project could beat a PD-1 inhibitor alone. A more relevant question for Summit, given its claims, might be whether ivonescimab can outdo a PD-1 plus a VEGF inhibitor like Avastin.
Chinese approval
The separate Harmoni-A data, presented at ASCO today, provide an opportunity for such a cross-trial comparison, namely against the Orient-31 trial of Innovent’s PD-1 inhibitor sintilimab plus the Avastin biosimilar Byvasda plus chemotherapy in EGFR-mutant NSCLC patients who had progressed after EGFR TKI therapy.
In Harmoni-A, at a 10 March 2023 cutoff date, ivonesimab plus chemo showed statistically significantly improved PFS over chemo alone, with medians of 7.1 versus 4.8 months respectively, giving a hazard ratio of 0.46 and a p value of less than 0.01.
These PFS numbers look similar to those seen in Orient-31.
Cross-trial comparison of inhibitors of PD-1 + VEGF + chemo
| Harmoni-A | Orient-31 | |||
|---|---|---|---|---|
| Ivonescimab + chemo | Chemo | Sintilimab + Byvasda + chemo | Chemo | |
| ORR | 51% | 35% | 44% | 25% |
| mPFS | 7.1mth | 4.8mth | 7.2mth | 4.3mth |
| OS | Immature | 21.1mth | 19.2mth | |
Source: ASCO 2024 & OncologyPipeline.
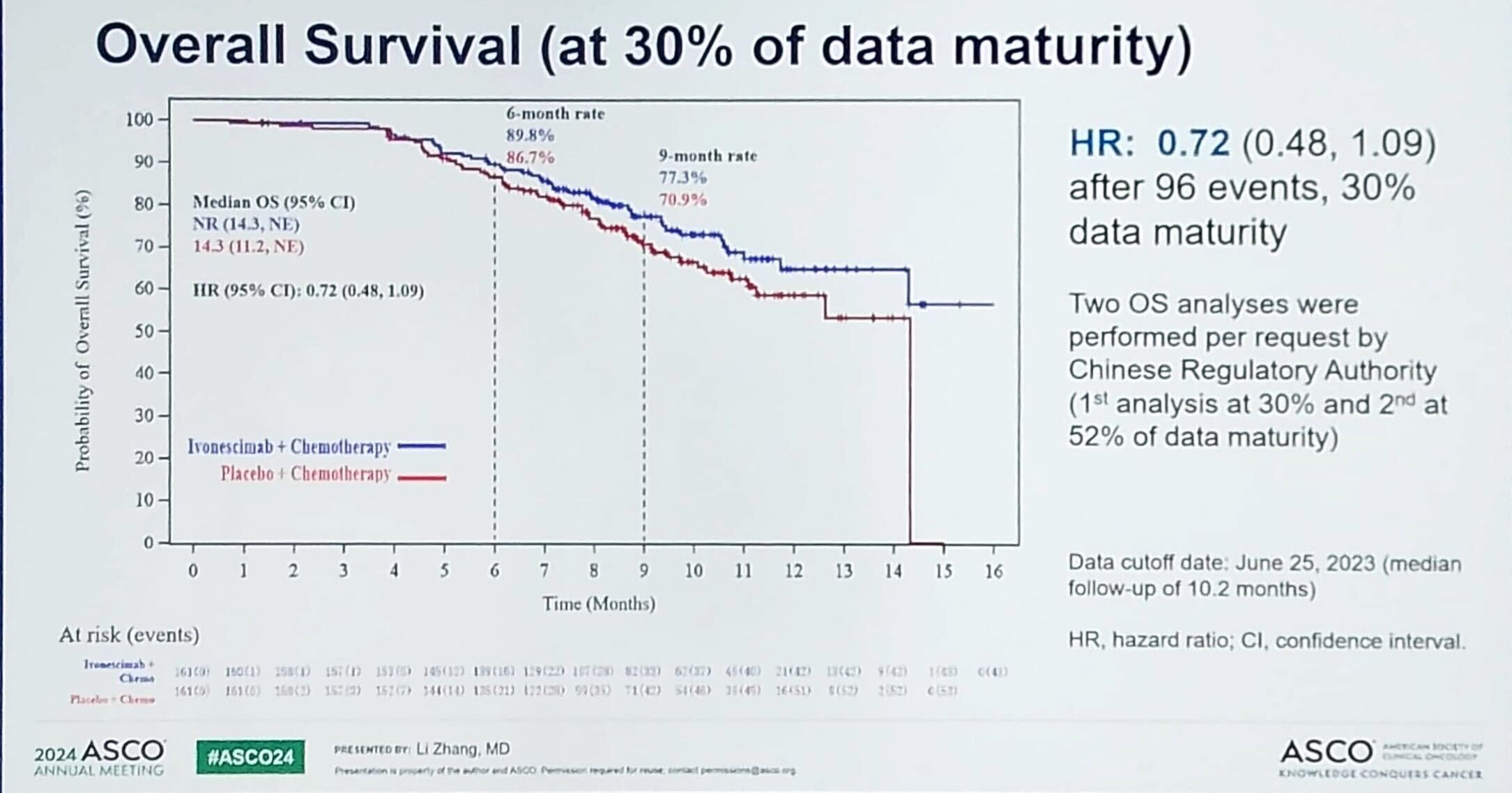
Overall survival data in Harmoni-A were immature, but a trend towards improvement with ivonescimab plus chemo was claimed, with a hazard ratio of 0.72 at 30% data maturity.
The Harmoni-A results were enough to get ivonescimab Chinese approval, Summit slipped into its press release yesterday. However, the FDA is unlikely to accept these data; to support US approval, Summit has begun enrolling US patients in Harmoni, the first of whom was recruited in May 2023.
The company also has a global phase 3 trial under way in first-line NSCLC, known as Harmoni-3.
Summit wasn’t the only group to benefit from its release yesterday. BioNTech, which has an anti-PD-L1 x VEGF bispecific, PM8002, saw its stock climb 6% on Thursday. That project will feature at ASCO in posters on Monday.
5057













