NACLC 2025 – BioNTech gets a gotistobart boost
The first stage of Preserve-003 shows a survival benefit in post-PD-(L)1 lung cancer – with caveats.
The first stage of Preserve-003 shows a survival benefit in post-PD-(L)1 lung cancer – with caveats.

BioNTech has claimed a win in the first stage of its phase 3 Preserve-003 trial in post-PD-(L)1 inhibitor non-small cell lung cancer, presented at the North America Conference on Lung Cancer on Saturday.
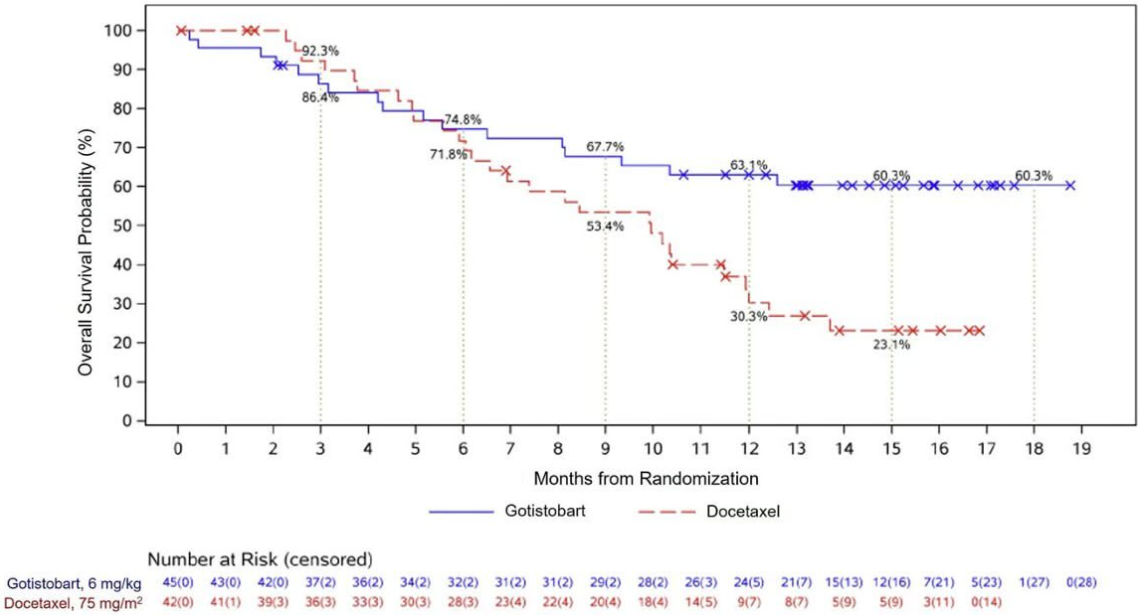
In this non-pivotal stage of the trial, median overall survival wasn’t reached with gotistobart, a next-gen anti-CTLA4 MAb, versus 10.0 months with docetaxel, at a cutoff date of 8 August. This represented a 54% reduction in the risk of death, with a hazard ratio of 0.46 and a nominal p value of 0.0102.
BioNTech previously gave a benchmark mOS of 9.4 months with docetaxel, based on the Tropion-Lung01 trial, so the control cohort of Preserve-003 doesn’t look to have underperformed.
These are impressive numbers in this tough-to-treat setting, but a closer look shows the survival curves crossing over: in the first few months there was a cohort of gotistobart-treated patients who died more quickly than those on docetaxel. These curves, which were widely shared on Twitter/X and confirmed by BioNTech, suggest that there are toxicities associated with gotistobart that could make it unsuitable for some patients.
Overall survival in patients with squamous NSCLC
Toxicity has long been a question for gotistobart, which BioNTech licensed from OncoC4 for $200m. The project is claimed to have a smart design, using a pH-sensitive molecule to avoid autoimmunity-related side effects that have dogged the likes of Bristol Myers Squibb’s Yervoy.
Despite this, high rates of adverse events and discontinuations were previously seen, for example in the Preserve-004 trial, testing gotistobart plus Keytruda in ovarian cancer.
In Preserve-003 BioNTech only disclosed that grade 3 or higher treatment-related adverse events were similar between the two cohorts, at 42% with gotistobart and 49% with docetaxel. But the NACLC presentation noted that 42% of gotistobart-treated patients had serious treatment-related AEs, versus 29% with docetaxel. Treatment-related AEs led to discontinuations in 13% of the gotistobart arm versus 5% of the control arm.
The most common adverse events with gotistobart were ALT increases (29%, 7% at ≥gr3) and diarrhoea (29%, 4% at ≥gr3).
There were also two treatment-emergent deaths in the gotistobart arm, from pneumonia and hemoptysis, although neither was deemed treatment-related. There were no deaths with docetaxel.
The results came in 87 patients with squamous disease who received 6mg/kg every three weeks. The trial’s first stage originally also enrolled non-squamous patients, but the study went on clinical hold in October 2024 with BioNTech citing “varying results” between squamous and non-squamous histologies.
The hold was lifted two months later, with the trial continuing in the squamous subtype only. However, the shape of the survival curves suggests that it won't be plain sailing for BioNTech in the squamous histology either.
The second, pivotal stage of Preserve-003 is now enrolling around 500 patients globally with squamous NSCLC, who will receive gotistobart at 6mg/kg or docetaxel. The primary endpoint is OS. The latest results could bode well for the next readout, expected in 2026 – if toxicity is kept in check. BioNTech's stock opened up 2% on Monday.
1270













