
SABCS 2025 – Pfizer could battle itself in first-line maintenance

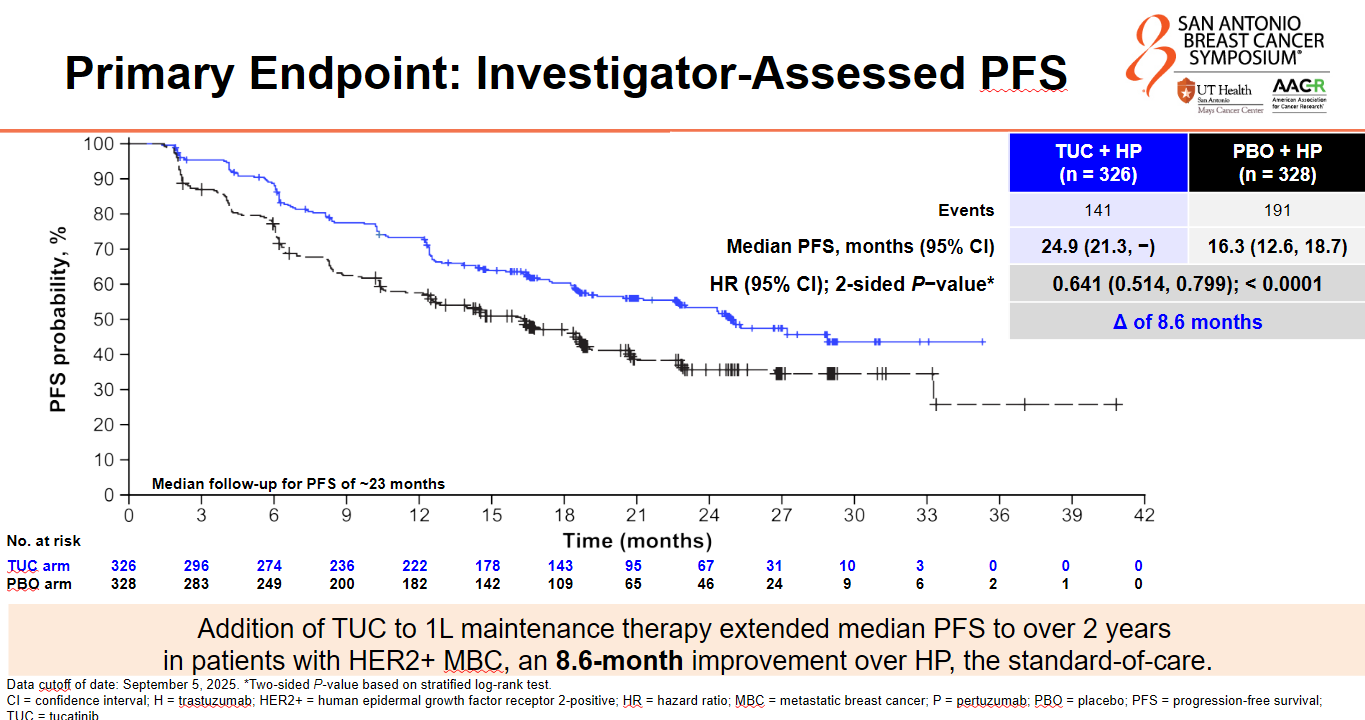
Pfizer’s small-molecule HER2 inhibitor Tukysa looks set to move into early HER2-positive breast cancer after showing a convincing benefit in first-line maintenance. The Her2climb-05 trial, presented at SABCS on Wednesday, found a 36% reduction in the risk of disease progression or death with Tukysa plus Roche’s Herceptin and Perjeta, versus Herceptin plus Perjeta, with a p value of less than 0.0001. Here, Tukysa could take on Pfizer’s CDK4/6 inhibitor Ibrance, which showed a similar relative benefit when added to standard of care in the Patina study; however, Patina only included patients with ER-positive disease, while Her2climb-05 enrolled ER-positive and negative patients. Still, the elephant in the room is AstraZeneca and Daiichi’s HER2-targeting ADC Enhertu, which recently impressed alongside Perjeta in Destiny-Breast09, in first-line HER2-positive disease. At a SABCS press conference, Dr Erika Hamilton of the Sarah Cannon Research Institute contended that “there will still be desire for a maintenance strategy, whether that’s Patina or Her2climb-05. I don’t think there’s going to be an appetite to continue Enhertu indefinitely.” An FDA decision on Enhertu in the front line is expected early next year; Pfizer has yet to disclose a filing for Tukysa, which is currently approved second line.
2627













