
Kura joins the pivotal menin push
The menin inhibitor battle shapes up with Kura, Syndax and J&J all now in phase 3.
The menin inhibitor battle shapes up with Kura, Syndax and J&J all now in phase 3.

Kura and Kyowa Kirin are awaiting FDA approval for their menin inhibitor ziftomenib in relapsed/refractory AML, but the big hope is for combinations, and here the phase 3 Komet-017 first-line trial has just been posted on clinicaltrials.gov.
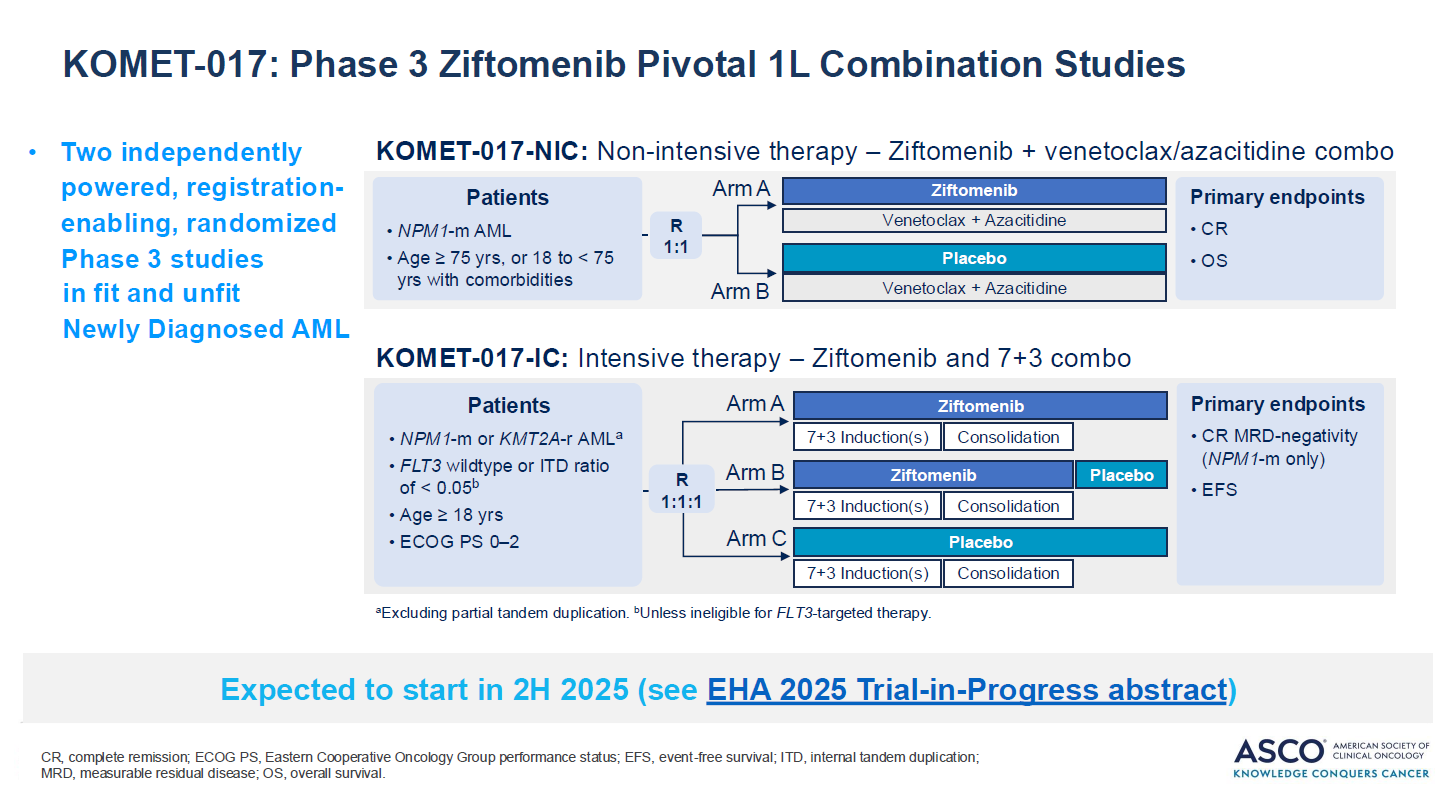
As previously disclosed, the protocol encompasses two freestanding studies: one in patients fit to receive intensive chemo, and one in chemo-ineligible subjects. In the chemo-ineligible setting Kura has been beaten to phase 3 by its menin rivals Syndax and Johnson & Johnson; however, in chemo-eligible patients, Kura is the first into a pivotal study, according to OncologyPipeline.
Syndax has disclosed pivotal plans in the chemo-eligible population, but as of its first-quarter earnings presentation in May these trials were still at the planning phase. Syndax’s chemo-ineligible phase 3 study, meanwhile, is investigator sponsored.
Phase 3 trials of menin inhibitors in first-line AML
| Project | Trial | Setting | Regimen | Primary endpoint | Timing |
|---|---|---|---|---|---|
| Ziftomenib (Kura) | Komet-017 | Chemo-eligible (NMP1m/KMT2Ar) | + 7+3 chemo | MRD-ve CR (NPM1m only, accelerated approval); EFS (full approval) | To start Sep 2025 |
| Chemo-ineligible (NMP1m only) | + Ven + aza | CR (accelerated approval); OS (full approval) | To start Sep 2025 | ||
| Bleximenib (J&J) | Camelot-2 | Chemo-ineligible (NPM1m/KMT2Ar) | + Ven + aza | CR; OS | Started Apr 2025 |
| Revuforj (Syndax) | Evolve-2 (HO177)* | Chemo-ineligible (NPM1m/KMT2Ar) | + Ven + aza | OS in NPM1m | Started Mar 2025 |
| Reveal-ND KMT2Ar | Chemo-eligible (KMT2Ar) | + 7+3 chemo | ? | “In planning phase” | |
| Reveal-ND NPM1m | Chemo-eligible (NPM1m) | + 7+3 chemo | ? | “In planning phase” |
*Investigator-sponsored trial. Source: OncologyPipeline & clinicaltrials.gov.
As outlined in February, chemo-ineligible patients in Komet-017, who must have NPM1 mutations, will receive ziftomenib plus Venclexta plus azacitidine, or ven/aza alone. Primary endpoints will be complete response rate, which could support accelerated approval, and overall survival, for full approval.
Meanwhile, chemo-eligible patients, whose cancers can have either NPM1 mutations or KMT2A rearrangements, will receive ziftomenib plus 7+3 chemo, versus chemo alone. This trial will also test ziftomenib versus placebo in the maintenance phase.
Primary endpoints will be MRD-negative complete response, to support accelerated approval, although an ASCO slide suggested that this would be in NPM1m patients only. Full approval could be supported by event-free survival.
The phase 1 Komet-007 tested these combos in first-line patients, with data reported at last year’s ASH meeting. Here, ziftomenib plus 7+3 chemo spurred complete responses in 91% of patients (100% in NPM1m and 83% in KMT2Ar subjects).
More data are due at the upcoming EHA meeting. Meanwhile, the first results with a front-line ven/aza combo are expected later this year.
Monotherapy
The battle for menin monotherapy approvals also continues. At ASCO Kura presented data from the Komet-001 trial of ziftomenib in relapsed/refractory NPM1m AML. Efficacy data looked very similar to those seen with Syndax’s Revuforj in this setting; however, Kura believes that its project could be differentiated on safety.
Although cases of QTc prolongation were disclosed in the ASCO abstract, Kura later noted that all three patients were on concomitant medications associated with QTc prolongation, and one had a prior diagnosis of atrial fibrillation.
This seemed to assuage some investor fears: Kura, which had looked like one of the early losers of the ASCO abstract drop, saw its stock climb 10% over the ASCO weekend.
Ziftomenib is due an FDA approval decision in relapsed/refractory NPM1m AML by 30 November. The project looks neck and neck with Syndax’s Revuforj, which was filed in the US for the same use in April. Revuforj is already approved in KMT2Ar relapsed leukaemia – but Kura has already given up on ziftomenib in this use.
However, at ASCO Kura’s chief medical officer, Mollie Leoni, told ApexOnco that in practice menin inhibitors wouldn’t be used as monotherapies, and she’s betting on ziftomenib being the most combinable. Kura now needs to prove this in phase 3.
1357













