
ASH 2025 – Newave shoots for a China first

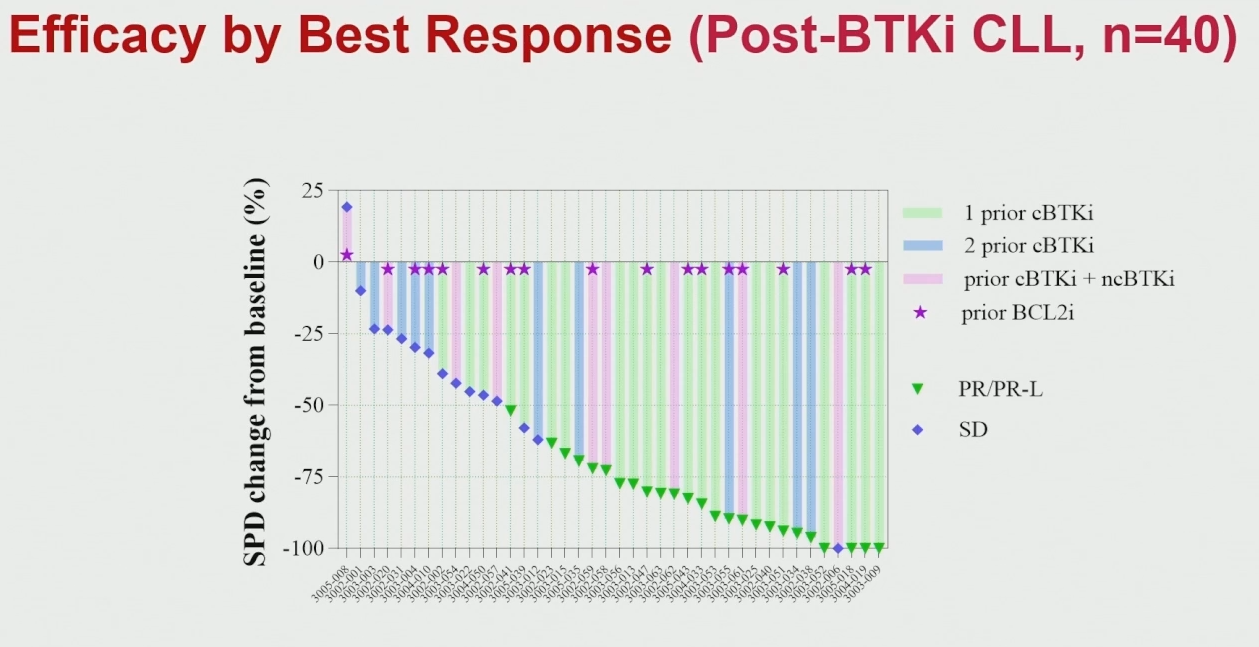
China’s Newave Pharmaceutical could soon become the first company to secure approval of a domestically discovered BTK inhibitor, having filed its lead project, rocbrutinib, with China’s NMPA in September. Data presented at ASH on Saturday suggest that this unusual molecule, said to inhibit BTK covalently under normal circumstances, but non-covalently if BTK carries the C481 mutation, might even have potential in patients who have failed covalent and non-covalent BTK inhibition alike. That’s a new development for rocbrutinib, which previously looked no better than non-covalents: at ASH two years ago no responses were reported among patients pretreated with a non-covalent BTK inhibitor like Lilly’s Jaypirca. The headline number from a phase 1 study now comprising 40 efficacy-evaluable CLL patients was a 63% response rate. This might seem unspectacular, but it was notable that best responses included four among eight patients pretreated with covalent and non-covalent BTK inhibitors, and two among four subjects who were triple-refractory (additionally having failed on a BCL-2 inhibitor). The presenter, Ohio State’s Dr Jennifer Woyach, suggested that this was especially relevant for such patients, who might have few treatment options. Coincidentally, Woyach is also lead investigator in the ASH presentation of Jaypirca’s Bruin CLL-314 study.
Rocbrutinib activity in relapsed/refractory CLL
2161













