
SABCS 2025 – Ember-3 brings no all-comers solace
Lilly reveals that an Inluriyo/Verzenio combo is being limited to ESR1-mutant disease.
Lilly reveals that an Inluriyo/Verzenio combo is being limited to ESR1-mutant disease.

After Lilly’s Inluriyo was, like Menarini’s Orserdu, approved only in ESR1-mutated breast cancer there was hope that combining the oral SERD with Verzenio could unlock the door to an all-comers population. That hope just evaporated, with Lilly revealing that a filing for Inluriyo plus Verzenio has also been limited to ESR1m patients.
This seems strange given that data from the supporting Ember-3 study, just revealed at the San Antonio Breast Cancer Symposium, appear to back the combo’s benefit in patients whose breast cancer is ESR1 wild type, as well as in those with ESR1m disease. Lilly told ApexOnco that its decision was based on feedback from the FDA.
Inluriyo plus Verzenio
Headline results from the SABCS dataset, from the registrational Ember-3 trial in second-line, ER-positive/HER2-negative breast cancer, hold little novelty value, given that they were disclosed once Inluriyo secured US approval in September. The trial enrolled all-comers, but its primary endpoint concerned PFS in ESR1m patients, and it’s to this subgroup that the FDA restricted approval.
So far so obvious; Inluriyo is an oral SERD (selective oestrogen receptor degrader), and the efficacy of most other oral SERDs has similarly been limited to ESR1m breast cancer.
However, last year’s SABCS offered Lilly hope, in the shape of a separate Ember-3 cohort, which gave patients Inluriyo in combination with the company’s approved CDK4/6 inhibitor Verzenio. That appeared to show a PFS benefit versus Inluriyo monotherapy that was independent of patients’ ESR1 status, though at the time the data were relatively immature.
SABCS on Friday brought mature data, with around three quarters of the patients involved having had a PFS event by an 18 August 2025 cutoff. Unlike the Inluriyo monotherapy cohort, whose all-comers activity was clearly driven by ESR1m patients, the combo beat Inluriyo monotherapy on PFS irrespective of whether patients’ breast cancer was ESR1m or not.
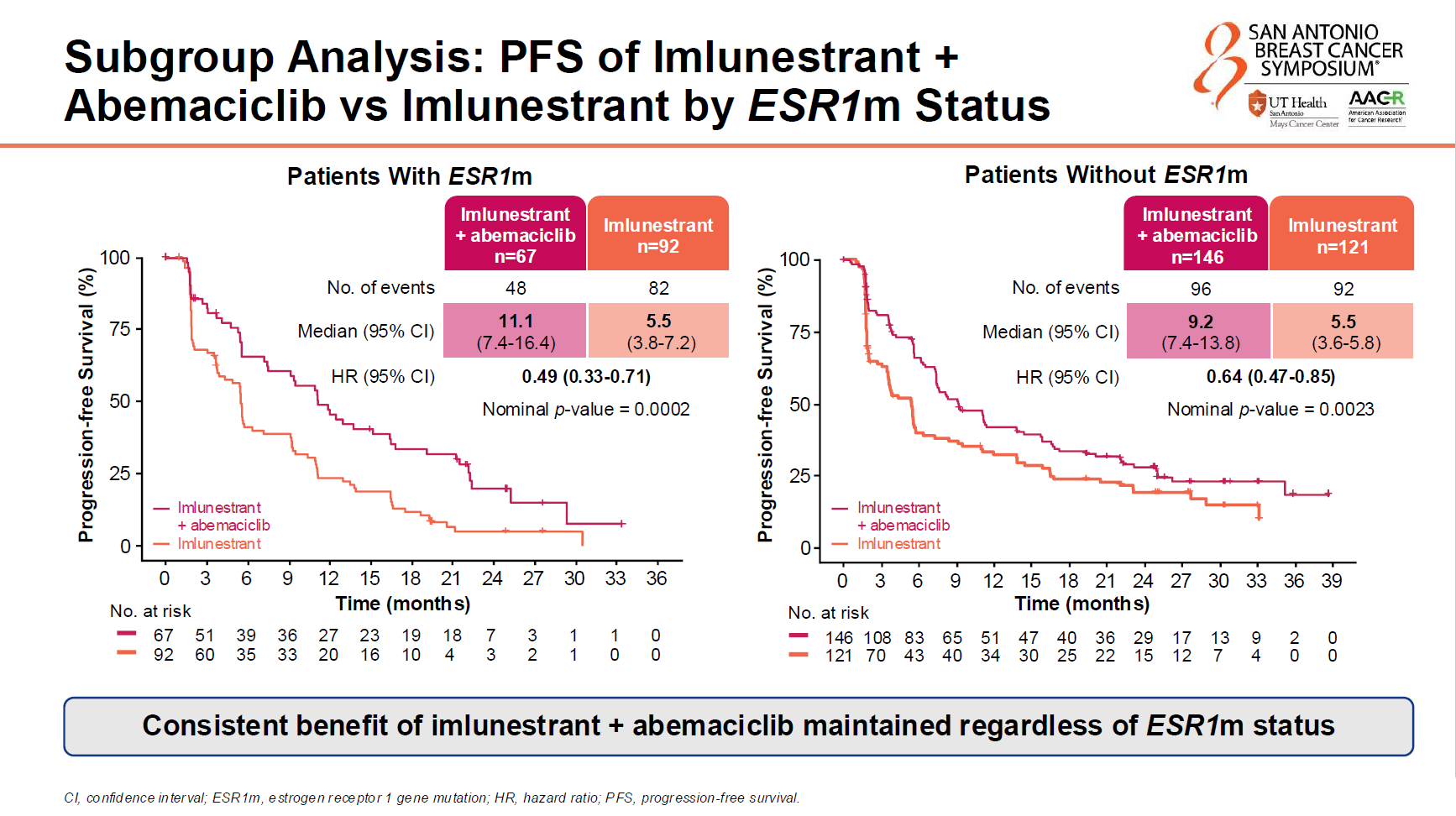
In spite of this apparent benefit in ESR1 wild-type disease, Lilly told ApexOnco: “We have submitted the combo for US regulatory review in ESR1-mutated metastatic breast cancer.”
Whatever reasons lie behind Lilly’s decision the development is important given the way this market is being cut. Most importantly, Roche appears to be gunning for an all-comers label for its oral SERD giredestrant, which it hopes to file next year.
AstraZeneca is pursuing a slightly different setting with camizestrant, seeking approval for patients who develop ESR1 mutations during first-line treatment but before disease progression. Meanwhile, Inluriyo followed Orserdu with an ESR1m-restricted monotherapy label, and Pfizer/Arvinas’s vepdegestrant has a PDUFA date (5 June 2026) for ESR1m disease.
Friday’s SABCS presentation did offer Lilly an unexpected positive for Inluriyo monotherapy in ERS1m patients: Ember-3’s secondary OS endpoint was earlier said to have failed to show a statistical benefit, but full data showed a median of 34.5 months versus 23.1 months for standard of care, a 0.60 hazard ratio and a p value of 0.0043.
The only reason this failed was that the vast majority of the powering in Ember-3 was saved for the PFS readout, and the SABCS presentation revealed that the bar to clear for OS was p=0.0000004.
2944













