AbbVie makes a bigger bet on SEZ6
But first it will need to identify the optimal dose in first-line small-cell lung cancer.
But first it will need to identify the optimal dose in first-line small-cell lung cancer.

AbbVie continues to back a target that few others have pursued, advancing turmetabart adizutecan, its SEZ6-directed ADC, from phase 1 directly into a pivotal study, according to a new listing on clinicaltrials.gov. This is a rapid move, given that the company has yet to find the optimal dose for this ADC.
Treading the risk/benefit line could be tricky. In phase 1 AbbVie evaluated four different dose levels, and the two highest, 3.0mg/kg and 3.5mg/kg, resulted in dose-limiting toxicities. At the ongoing World Conference on Lung Cancer the company identified 1.8mg/kg every three weeks as the recommended phase 2 monotherapy dose, but it has not yet shared combination data with budigalimab, its PD-L1 antibody, which underpin the rationale for the new pivotal trial in first-line small-cell lung cancer.
That study, called Sezanne, is set up initially to test two different turmeta-A doses in combination with Tecentriq against the current standard of care, Tecentriq plus chemotherapy. Once the optimal regimen is determined, the phase 3 portion will launch, with overall survival as the primary endpoint.
For context, Tecentriq’s Impower-133 study, which secured its approval in the first-line setting, achieved a 60% ORR and 5.2 months of median PFS.
Early-stage data
AbbVie abandoned its earlier SEZ6-targeting ADC, ABBV-011, inherited from Stemcentrx, but data from a phase 1 trial of turmeta-A (previously known as ABBV-706) appear to have boosted confidence in the target.
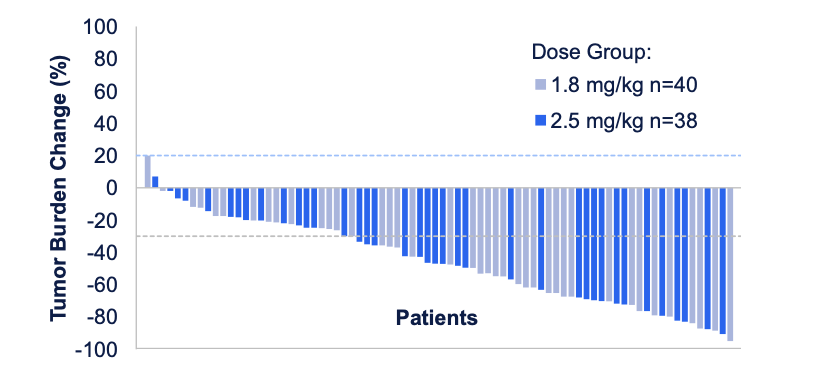
At World Lung, AbbVie reported that the ADC delivered a 58% ORR and a median PFS of 5.7 months in 80 relapsed/refractory SCLC patients treated at the lowest doses, 1.8mg/kg and 2.5mg/kg. ORRs were similar between doses, at 56% and 59% respectively, while median PFS was 6.8 months with 1.8mg/kg and 5.6 months with 2.5mg/kg.
These seem likely to be the doses that AbbVie will advance into phase 3, although there are early signs that 1.8mg/kg could emerge as the preferred option. Grade 3 or higher treatment-related adverse events occurred in 49% of patients receiving this dose, versus 77% in the 2.5mg/kg group. Still, 10% of patients in the lower-dose group discontinued, versus 8% receiving the higher dose.
Anaemia and neutropenia topped the list of serious side effects, and seven patients developed interstitial lung disease, with four of these at grade 3 or above. This will be a key safety metric to watch as the program moves into phase 2/3.
Turmetabart adizutecan in phase 1
AbbVie’s gamble comes as other players step up efforts in SCLC. PharmaMar/Jazz’s Zepzelca, which is included as an optional treatment in the comparator arm of Sezanne, is under regulatory review in the US for first-line extensive-stage SCLC maintenance, with a PDUFA date set for October 7.
Amgen is conducting multiple phase 3 trials of Imdelltra in various first-line settings, and the DLL3 bispecific already holds accelerated approval in relapsed/refractory SCLC based on a 40% ORR in the Dellphi-301 trial.
Meanwhile, BioNTech is advancing pumitamig in the phase 3 Rosetta Lung-01 in first-line ES-SCLC induction and maintenance, after reporting confirmed ORR of 76%, at the 20mg/kg dose, at World Lung.
1982