ESMO 2025 – bemarituzumab fails to convince
Amgen and Zai Lab’s FGFR2b antibody shows no benefit at the two-year mark.
Amgen and Zai Lab’s FGFR2b antibody shows no benefit at the two-year mark.

Amgen’s $1.9bn purchase of Five Prime Therapeutics in 2021 is starting to look like an expensive mistake. Through that acquisition Amgen acquired bemarituzumab, whose first phase 3 results were described as “not practice changing” by an ESMO discussant, Memorial Sloan Kettering’s Dr Yelena Janjigian.
In June, Amgen and its partner Zai Lab reported that the Fortitude-101 first-line gastric cancer trial had met its primary endpoint of overall survival, but in September, Amgen disclosed that the trial had shown a decreased overall survival benefit at a later analysis. The partners are now awaiting data from a second phase 3, Fortitude-102, before deciding whether to file; the update hit Zai Lab particularly hard, sending that company’s stock down 6% on the day of the announcement.
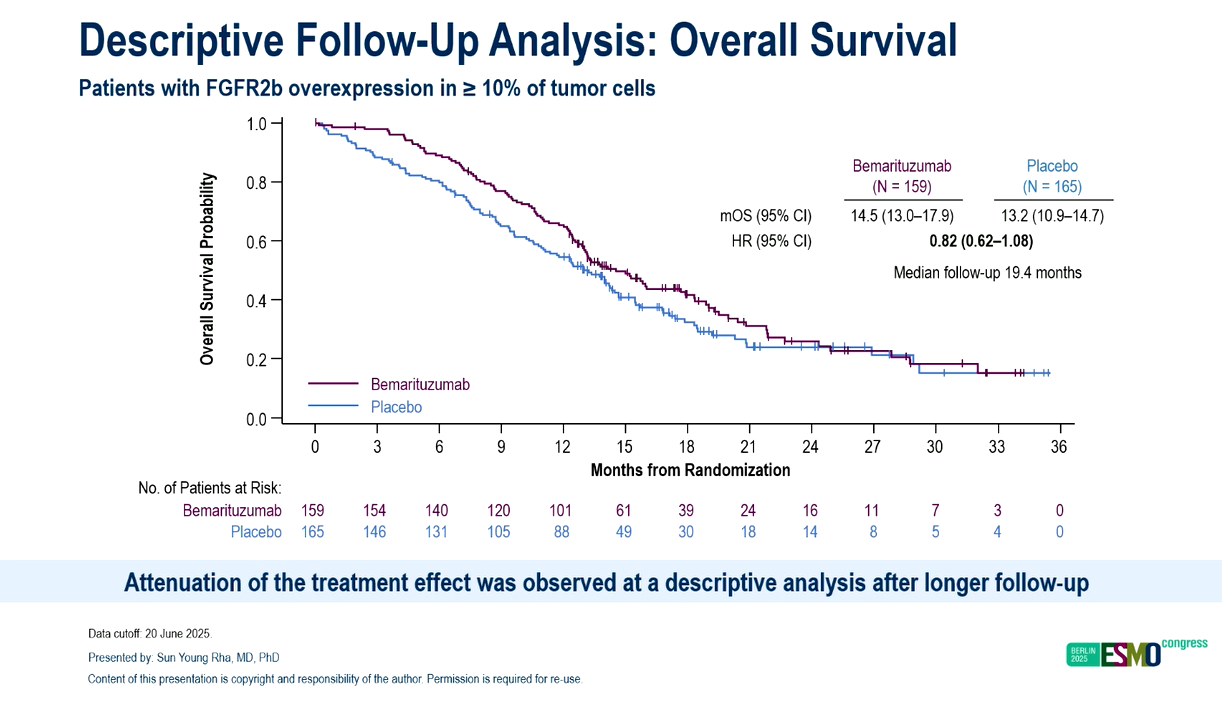
Full data were presented during the third presidential symposium at ESMO on Monday from Fortitude-101, which initially enrolled first-line gastric or GEJ patients with any FGFRb2 overexpression, later amended to 10% or higher expression.
At the primary analysis, median overall survival reached 17.9 months with bemarituzumab plus chemotherapy, versus 12.5 months for chemotherapy alone, giving a hazard ratio of 0.61, and a p value of 0.005. Yet, the follow-up was less than a year, and with an additional 7.6 months, the survival curves converged. By the two-year mark, no meaningful difference remained.
The discussant, Janjigian, suggested that late censoring and biomarker heterogeneity could be factors behind the apparent waning of bemarituzumab’s effect.
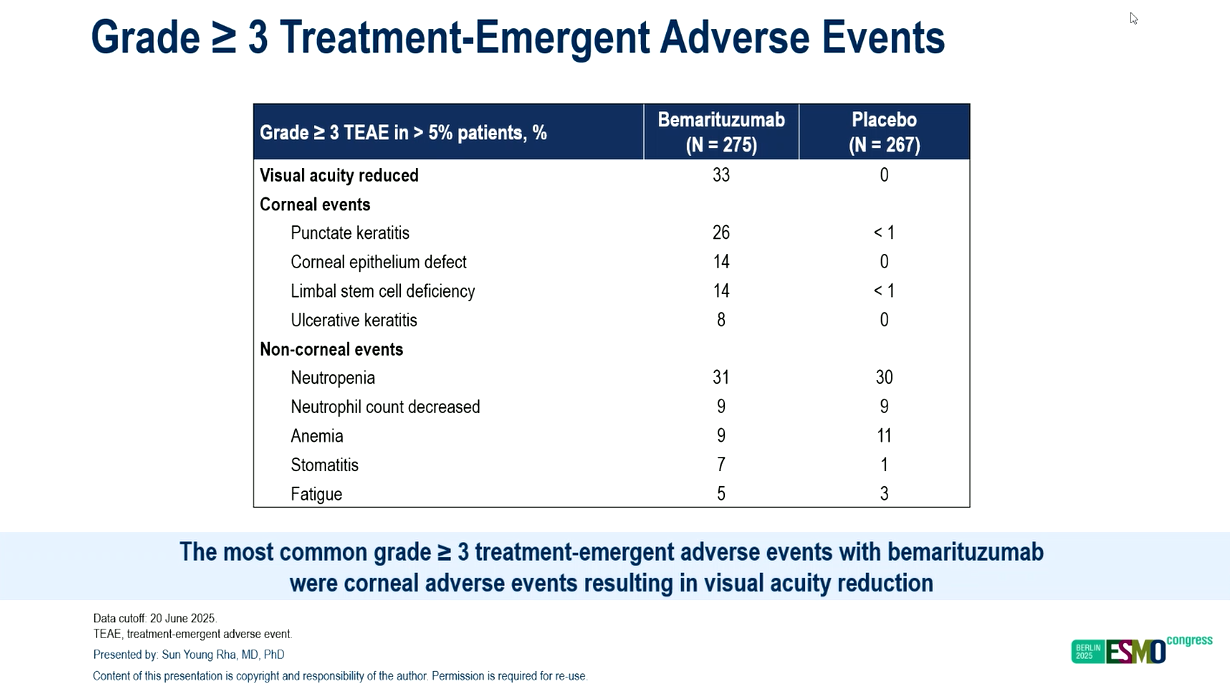
Tolerability has also been a sticking point. Ocular toxicities, a characteristic side effect of the FGFR2b antibody, led to treatment discontinuation in 28% of patients and might have further contributed to the diminishing survival advantage.
Trial design choices also drew scrutiny. Only patients who had completed a single cycle of chemotherapy were eligible, and the control arm lacked PD-(L)1 therapy, the standard of care in this indication.
For now, Amgen’s and Zai Lab’s hopes hinge on Fortitude-102. That trial, also in first-line gastric cancers with 10% or higher FGFR2b expression, is testing bemarituzumab plus Opdivo plus chemo, versus Opdivo alone, so could be more representative of current practice.
Furthermore, clinicians have become more used to dealing with eye toxicities, Janjigian noted, adding that, because of this, ocular tox might be less of a problem in Fortitude-102. Data from this trial are due by the end of this year or early next year.
3250