
ESMO 2025 – Lilly overshadows Astra’s folate reveal
The battle intensifies as LY4170156 matches torvuta-S with activity in FRα <25% expresers.
The battle intensifies as LY4170156 matches torvuta-S with activity in FRα <25% expresers.

With the ESMO presentation on AstraZeneca's anti-folate receptor alpha ADC torvutatug samrotecan largely rehashing data already revealed in its abstract, it was left to Lilly to deliver the meeting’s surprise in treating ovarian cancer with this mechanism of action.
This came courtesy of a low-key ESMO poster, which quietly revealed that Lilly’s own FRα-directed ADC, LY4170156, had delivered a response rate of 40% in patients whose cancers expressed FRα at levels of 24% down to 0%. This is the first time such an important disclosure has been made, and positions LY4170156 as a key future competitor against AbbVie's approved anti-FRα ADC, Elahere.
That’s not to say that Astra’s torvuta-S has been eclipsed. That asset too is active at low FRα expression levels, ESMO citing ORRs of 61% ORR in tumours with ≥75% FRα expression, and 48% in those with levels below 25%, in the first-in-human phase 1/2 Fontana study in platinum-resistant ovarian cancer. Across all 168 patients in its ESMO presentation given 1.6-2.4mg/kg the ORR was 54%.
Ocular toxicity is a key drawback of Elahere, but in Fontana it was seen in 15% of patients, and at grade 3 or above in just one subject. However, while the ESMO abstract promised the first PFS data for torvuta-S, none was provided by the presenter, Dr Ana Oaknin of Vall d'Hebron Institute of Oncology.
Lilly disclosure
Instead, the low-key Lilly disclosure appears to be the bigger ESMO story in FRα. Until now all this company had revealed was LY4170156’s activity in FRα cuts of above or below 75%, most recently at ASCO.
The ESMO poster concerned 104 efficacy-evaluable patients, up from the 58 detailed at ASCO. Across all 104 subjects the ORR stands at 50%.
First-in-human data for LY4170156
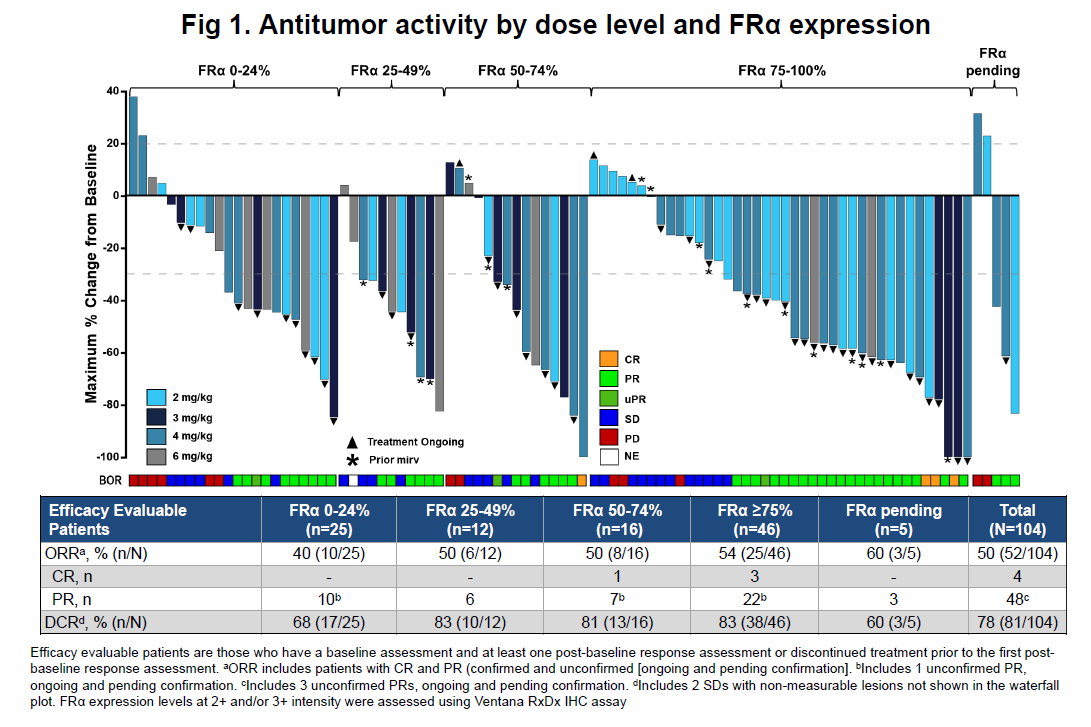
Activity in cancers with low FRα expression is a key battleground, given that Elahere is approved only for patients with levels of ≥75%, where the label cites a 42% ORR.
That’s why until recently competitors had been cutting their datasets at the 75% level. Astra, and now Lilly, showing activity in tumours with FRα expression of 25% and below shows how this battle for market expansion is intensifying.
Such comparisons are relevant not only for AbbVie, which gained Elahere through the $10bn acquisition of ImmunoGen, but also for Genmab, which shelled out $1.8bn for ProfoundBio and its anti-FRα ADC rinatabart sesutecan. For its part, Lilly gained LY4170156 through the acquisition of Mablink for an estimated $250m, while torvuta-S appears to be an internal Astra project.
Genmab’s rina-S boasts a 55% ORR in the Rainfol-01 trial, but here again no details have been provided backing activity in patients with very low FRα expressers. A waterfall plot presented at this year’s Society of Gynecologic Oncology meeting suggested that this molecule too might struggle with activity at low expression levels: ORR among 13 patients with FRα ≥75% was 69% but in 23 with FRα <75% it was just 30%.
Lilly’s LY4170156 is about to enter pivotal development on the basis of its phase 1 data alone: the phase 3 Framework-01 study, which will test an Avastin combo versus Elahere or Avastin, is due to begin in November. Astra has yet to reveal pivotal plans for torvuta-S.
Cross-trial comparisons for anti-FRα ADCs
| Project | Company | Trial | ORR in all-comers | ORR in FRα ≥75% | ORR in FRα <75% | ORR in FRα <25% |
|---|---|---|---|---|---|---|
| Elahere | AbbVie (ex ImmunoGen) | Mirasol | 42% (n=225) | 42% (n=225) | Not tested | Not tested |
| Rina-S | Genmab (ex ProfoundBio) | Rainfol-01 | 38% (n=40) | 68% (n=13) | 30% (n=23) | Not split out |
| LY4170156 | Lilly (ex Mablink) | LOXO-FRA-24001 | 50% (n=104) | 54% (n=46) | 45% (n=53) | 40% (n=25) |
| Torvuta-S | AstraZeneca | Fontana | 53% (n=168) | 61% (n=56) | Not split out | 48% (n=61) |
Source: OncologyPipeline.
3785













