Immatics’ PRAME gift keeps on giving
The company dribbles out more data on IMA203, and the numbers head in the right direction.
The company dribbles out more data on IMA203, and the numbers head in the right direction.

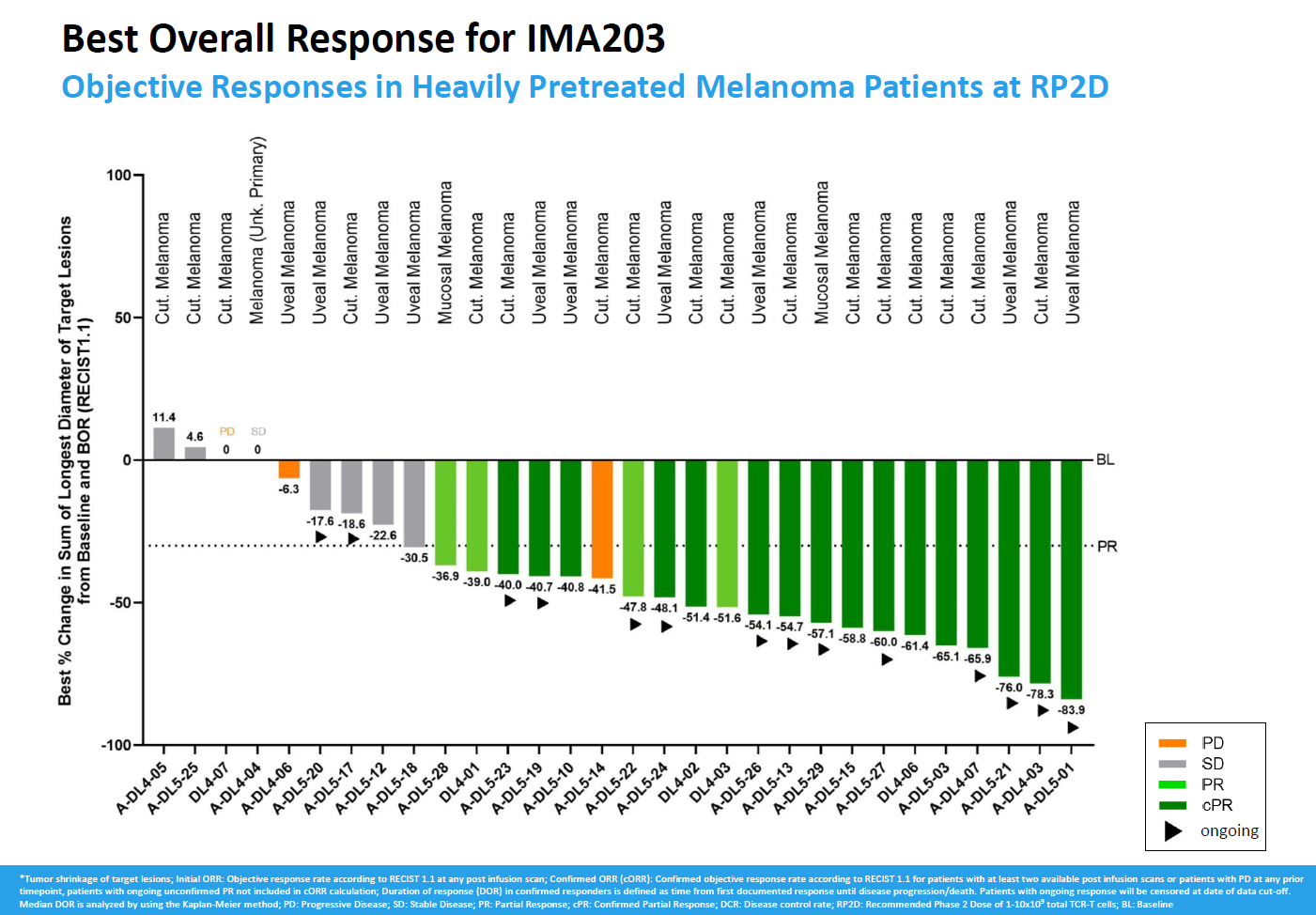
With more patients treated in a phase 1 study of Immatics’ anti-PRAME T-cell receptor project IMA203, efficacy is holding up – and then some. The company today revealed a confirmed ORR of 55% among 29 evaluable melanoma patients, an improvement on the 50% from the first 12 reported last November.
The numbers refer to the recommended phase 2 dose of IMA203, and this appears to have overcome Immatics’ problem of initial remissions melting away as patients relapsed. Still, having touted early promise in several cancers the focus for IMA203 is now firmly on melanoma, with a phase 2/3 trial in second-line or later cutaneous melanoma, and possibly uveal melanoma, to start in the fourth quarter.
IMA203 is actually one of three separate projects Immatics has in the pipeline that target PRAME (preferentially expressed antigen of melanoma). In November the group reported the first efficacy data for IMA203CD8, which comprises IMA203 with an added CD8 co-receptor to allow CD4+ T cells to be utilised, and those data – 56% confirmed ORR in nine melanoma subjects – still stand.
Earlier it moved into the clinic IMA402, a bispecific T-cell engaging MAb targeting PRAME, insisting that the two distinct modalities could be developed side by side. Today it promised to deliver the first clinical data with IMA402, in at least 15 patients with an initial focus on melanoma, in the second half of 2024.
Melanoma in focus
As for the IMA203 phase 1 dataset, Immatics is zeroing in on those melanoma patients in a phase 1a portion who were given dose levels 4 or 5 (1 to 10 billion TCR-positive T cells), plus all those from a subsequent cohort that used only this level, which it calls the recommended phase 2 dose.
30 subjects are now evaluable, and 22 of these yielded initial tumour reductions meeting the criteria for a partial response. 16 of those responses have been confirmed, five have relapsed, while one remains ongoing but hasn’t yet been confirmed, resulting in a topline ORR number of 55% with a denominator of 29 patients.
Last November’s 50% confirmed ORR was based on an initial 62% rate, including unconfirmed responses. That, and today’s update, thus represent a meaningful improvement from the first reported IMA203 dataset, where just five of 13 responses ended up being confirmed; that comprised melanoma, but activity was also seen in synovial sarcoma and head and neck cancer.
Immatics says it has now treated 65 patients with IMA203 across all dose levels and tumour types, but isn't saying what the efficacy is like beyond melanoma. It highlights the absence of IMA203-related deaths among the 65 patients, though liver enzyme elevations have been noted: grade 3+ ALT increases have been seen in 9% of subjects, and grade 3+ AST increases in 7%.
A fuller clinical update is expected at a second-half medical conference – ESMO seems possible – and an update on the next-generation TCR asset IMA203CD8 is expected in a similar timeframe.
After closing up 9% yesterday Immatics traded up another 7% in today. A lot is riding on the company in part because it listed via Spac (special-purpose acquisition company), and with the shares some 20% above their $10 issue price it remains one of biotech's few Spac success stories.
1809