
ASCO 2024 – AstraZeneca’s GPC3 secret sauce
A Car-T therapy to which Astra has rights has wowed in a solid tumour after others had disappointed.
A Car-T therapy to which Astra has rights has wowed in a solid tumour after others had disappointed.

The intrigue surrounding AbelZeta’s solid tumour Car-T breakthrough went up a notch today with the revelation that a more recent cut of the liver cancer trial of this project, C-CAR031, has seen the confirmed response rate climb from 50% to 57%.
The 50% ORR, relating to a January cutoff described in the abstract, had already impressed, given that patients had progressed on a median 3.5 prior therapy lines. With AstraZeneca’s AZD5851 using the same construct – the two companies have a cross-licensing arrangement – investors and other Car-T players in this space will want to know why C-CAR031 has shown promise where others failed.
This divergence has been thrown into focus by the failure of Noile-Immune’s similarly acting Car-T therapy NIB102 to yield a single response at ASCO, and being discontinued by partner Takeda. Other players working on anti-GPC3 Cars include Sotio, Legend and the private US biotech Eureka Therapeutics.
Construct not target?
One possible reason for AbelZeta’s success is the construct C-CAR031 uses, suggesting that simply targeting the GPC3 protein without additional bells and whistles isn’t enough.
At today’s ASCO presentation Dr Qi Zhang, from Zhejiang University School of Medicine, revealed that C-CAR031 used an affinity-tuned antibody-derived binding domain that he suggested might enhance safety.
Meanwhile, an “armouring” element on C-CAR031 comprises a co-expressed dominant-negative TGFβ receptor that’s been truncated, so it lacks an intracellular domain necessary for downstream signalling. This feature is designed to protect the Car-T cells from TGFβ-driven immunosuppression.
That said, serious treatment-related adverse events in AbelZeta’s study were experienced by 38% of patients, though among those of special interest Zhang highlighted a mere 4% rate of grade 3 cytokine release, and no neurotoxicity of any severity.
He presented a waterfall plot on 23 efficacy-evaluable patients (arguably this should also include another subject, who was excluded following a “major protocol deviation”), showing tumour shrinkage in all but two of them. As of a 14 March cutoff there are 13 confirmed partial responses.
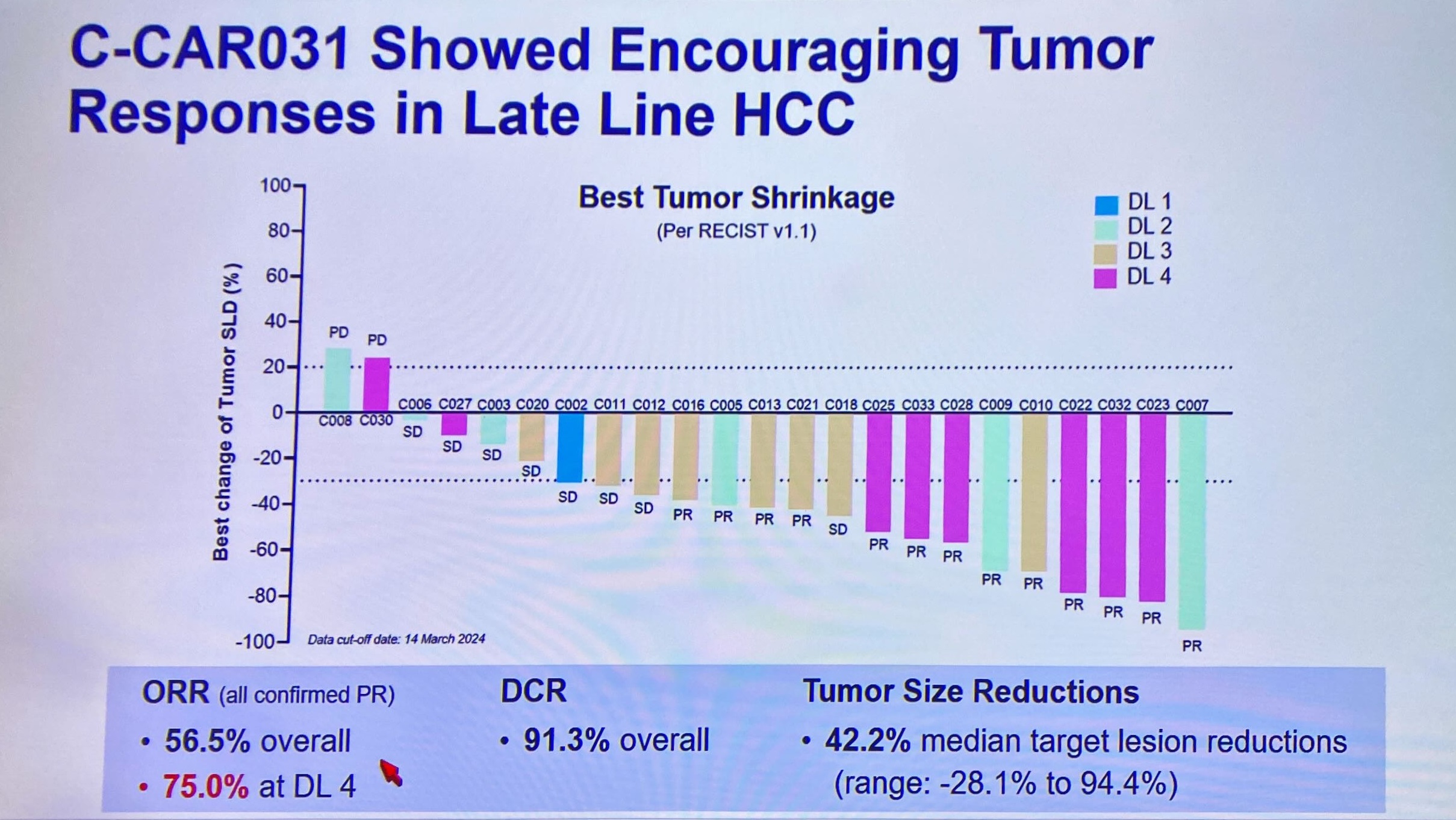
The responses were seen across various dosing levels, but Zhang highlighted six among eight subjects given the highest C-CAR031 dose. Across all patients six responders relapsed, but at nine months’ median follow-up the median response duration stands at over seven months.
A search of OncologyPipeline reveals numerous clinical-stage GPC3-targeting Car-T projects, many at Chinese companies, though only some of these appear to have additional features beyond GPC3 targeting.
Meanwhile, preclinical Car-T assets include an unnamed Crispr/Cas9-edited project at Crispr Therapeutics, the result of a collaboration with Roswell Park Comprehensive Cancer Center, and Gilead’s KITE-509; the latter is also said to block the TGFβ signal, but its development doesn’t appear to be a priority.
Under a deal struck last December AbelZeta, formerly known as Cellular Biomedicine Group, obtained a milestone and royalty interest in Astra’s AZD5851, which uses the same construct as C-CAR031. Meanwhile, Astra secured China co-development rights to C-CAR031.
A key question now is whether the impressive data AbelZeta has generated in China can be reproduced in the west, by a global company and in a western population. The first patient in AZD5851’s clinical trial was dosed recently, and first human data are eagerly awaited.
Selected clinical-stage Car-T projects targeting GPC3
| Project | Company | Status | Note |
|---|---|---|---|
| C-CAR031 | AbelZeta Pharma | ASCO data showed 57% cORR in GPC3+ve liver cancer | ”Armoured” with dnTGFbRII |
| AZD5851 | AstraZeneca | Athena trial in GPC3+ve liver cancer | ”Armoured” with dnTGFbRII |
| BOXR1030 | Sotio | Duet-1 trial in GPC3+ve solid tumours | Co-expresses glutamic-oxaloacetic transaminase 2 (GOT2) |
| ECT204/ JWATM204 | Eureka Therapeutics/ JW | Ph1 in liver cancer | Apparently not GPC3 preselected |
| LB2101 | Legend Biotech | Ph1 in GPC3+ve liver cancer | Said to use immunosuppressive TME signal to augment potency |
| Ori-C101 | OriCell Therapeutics | Beacon trial in GPC3+ve liver cancer | – |
| CT011 | Carsgen Therapeutics | Ph1 in GPC3+ve liver cancer | Company also has anti-GPC3 Cars CT0180 & CT0181, using a different technology, in investigator-sponsored trials |
| EU307 | Eutilex | Ph1 in GPC3+ve liver cancer | – |
| GPC3 CART | Shanghai Genechem | Ph1/2 in GPC3+ve liver cancer | – |
| GPC3-CAR-T | BriSTAR Immunotech | Investigator-sponsored trial in GPC3+ve liver cancer | – |
| IM83 CAR-T | Beijing Imunopharm | Ph1 in GPC3+ve liver cancer | – |
| NIB102/ TAK-102 | Noile-Immune | ASCO data showed 0% ORR in GPC3+ve solid tumours | Discontinued by Takeda; co-expresses IL-7 & CCL20 |
Source: OncologyPipeline.
8990













