
Immatics’ PRAME T-cell engager improves
The response rate jumps to 30% with higher doses of IMA402.
The response rate jumps to 30% with higher doses of IMA402.

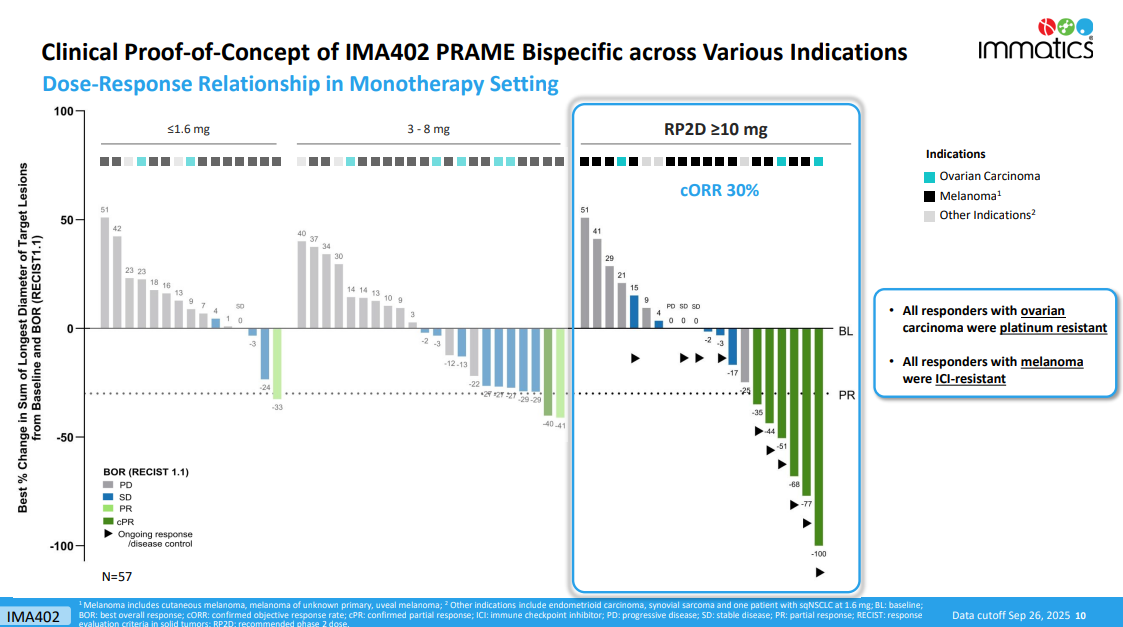
Last year, Immatics failed to impress with its PRAME-targeting T-cell engager IMA402, but the company is back with updated phase 1 results that cast the project in a better light. Specifically, Immatics last year reported a 5% ORR among 21 patients receiving 0.02-4mg, while on Wednesday it revealed a 30% confirmed ORR among 20 patients receiving 10-30mg.
Immatics also presented more results with its anti-MAGEA4/8 T-cell engager IMA401, which appear to confirm that project’s promise, despite it previously being abandoned by Bristol Myers Squibb. However, the main focus for this asset is as part of a combination with IMA402.
As well as evaluating the IMA402/IMA401 combo, initially in squamous NSCLC, Immatics will also pursue IMA402 in cutaneous melanoma and gynecological cancers. The first step will be confirming the go-forward IMA402 dose: Immatics will evaluate two doses within the 10-30mg range in phase 1b expansion cohorts; it believes that these cohorts could later be converted to phase 2 trials, which could then support regulatory filings.
The group also disclosed plans to test IMA402 in first-line melanoma, in combination with checkpoint inhibitors, and in platinum-sensitive ovarian cancer, alongside standard of care.
Late line
The latest IMA402 data, however, have come in heavily pretreated solid tumour patients, with a median of three prior therapies.
At a cutoff date of 26 September, 80 patients had been treated with various doses of IMA402; 20 of these received 10-30mg, and were efficacy evaluable.
Among this group there were six responses, four in melanoma, and two in ovarian cancer. Notably, all the melanoma patients were checkpoint inhibitor resistant, and all the ovarian patients were platinum resistant.
In comparison, in post-checkpoint inhibitor melanoma, Iovance’s TIL therapy Amtagvi has produced an ORR of 32%, according to its label – but this number is likely flattered by patient exclusions.
IMA402 activity in phase 1
Immatics described IMA402 as well tolerated; however, there were two dose-limiting toxicities, both at lower doses tested, 0.08mg and 0.3mg. The first was a grade 4 cytokine release syndrome, which the company put down to a high starting dose. It’s since modified its dosing steps, and said it hasn’t seen any more grade 4 CRS.
The other DLT was a grade 3 renal adverse event, but Immatics execs noted on a conference call that the patient had come into the trial with an existing renal injury.
Cell therapy vs bispecific
Immatics is also developing a PRAME-targeting engineered T-cell receptor, anzutresgene autoleucel (anzu-cel, previously known as IMA203). That has produced an ORR of 56% in heavily pretreated melanoma, and is currently in the phase 3 Suprame trial in relapsed cutaneous melanoma, with Immatics recently starting a phase 2 uveal melanoma cohort in its phase 1/2 trial.
Immatics noted that, given that cell therapy is more arduous to administer, it sees anzu-cel mainly being used in later lines, while IMA402 could be more suited to earlier lines; as well as front-line metastatic disease, the company also raised the possibility of going into adjuvant and neoadjuvant settings with the T-cell engager.
However, Immatics believes that IMA402 could also have utility in later lines, and reckons having two different modalities aimed at the same target could give it “versatility”.
Investors seemed inclined to agree, with Immatics’ stock closing up 16% on Wednesday.
2873













