
Kineta is down, but not yet out

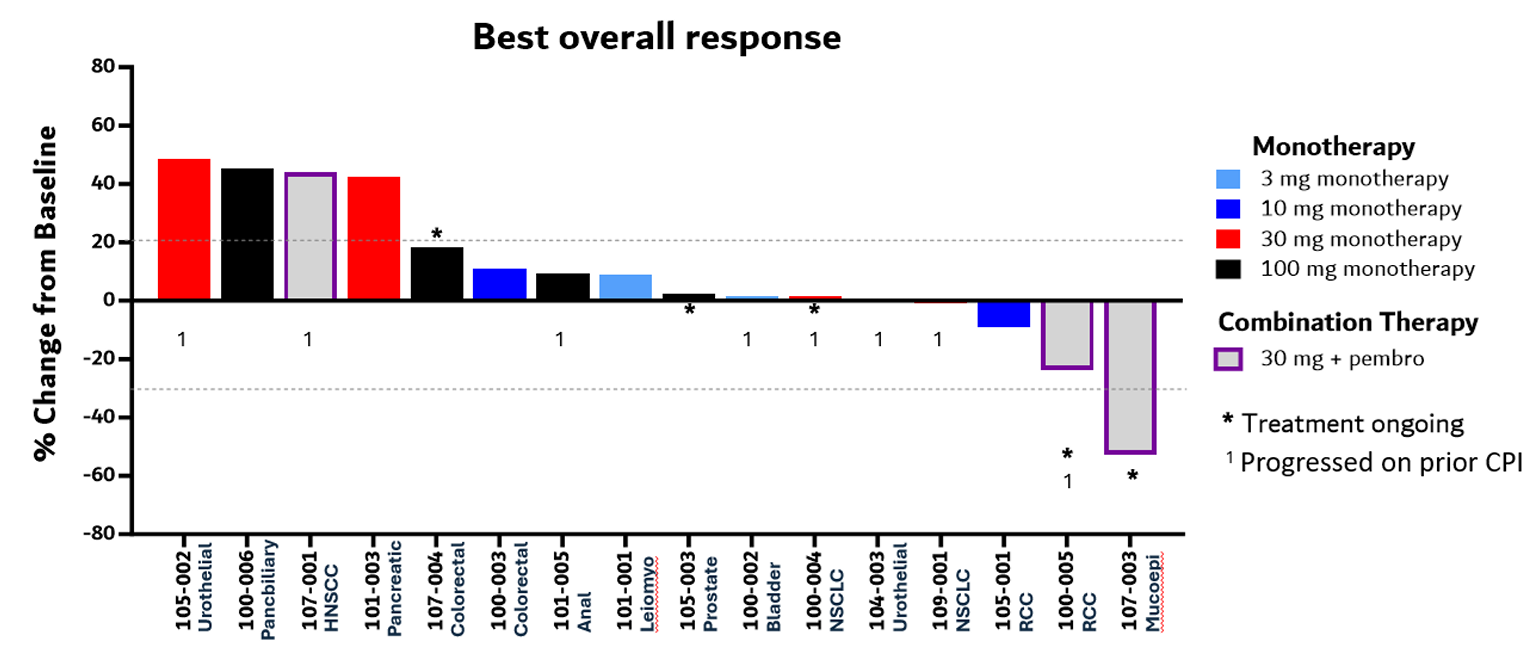
Unable to raise cash from investors, tiny Kineta has seen its market cap dwindle to just $8m, but the company isn’t done yet. Monday brought news that it was restarting patient screening for enrolment into the phase 1/2 trial of its anti-VISTA MAb KVA12123 – a study that six months ago it moved to wind down. Kineta’s change of heart has been brought about by a recent deal with TuHura, which paid $5m for an option over a formal licensing arrangement covering KVA12123. Kineta says the February decision to wind down KVA12123 was driven simply by lack of funds, after some investors who had agreed to put up private money withdrew their support. Now, armed with an extra $5m, Kineta can again fund its study, and will presumably hope that it can generate sufficient promising data to tempt TuHura into a full licensing deal. TuHura is a private US biotech that until December had been known as Morphogenesis, and subject to an extension it has until 1 October to decide whether to opt in. Apart from KVA12123 the industry’s only other clinical-stage anti-VISTA projects, according to OncologyPipeline, are Sensei’s SNS-101, PharmAbcine’s PMC-309 and Hummingbird's HMBD-002.
Phase 1 efficacy data for KVA12123
766













