
Talzenna's US limit looks set to remain

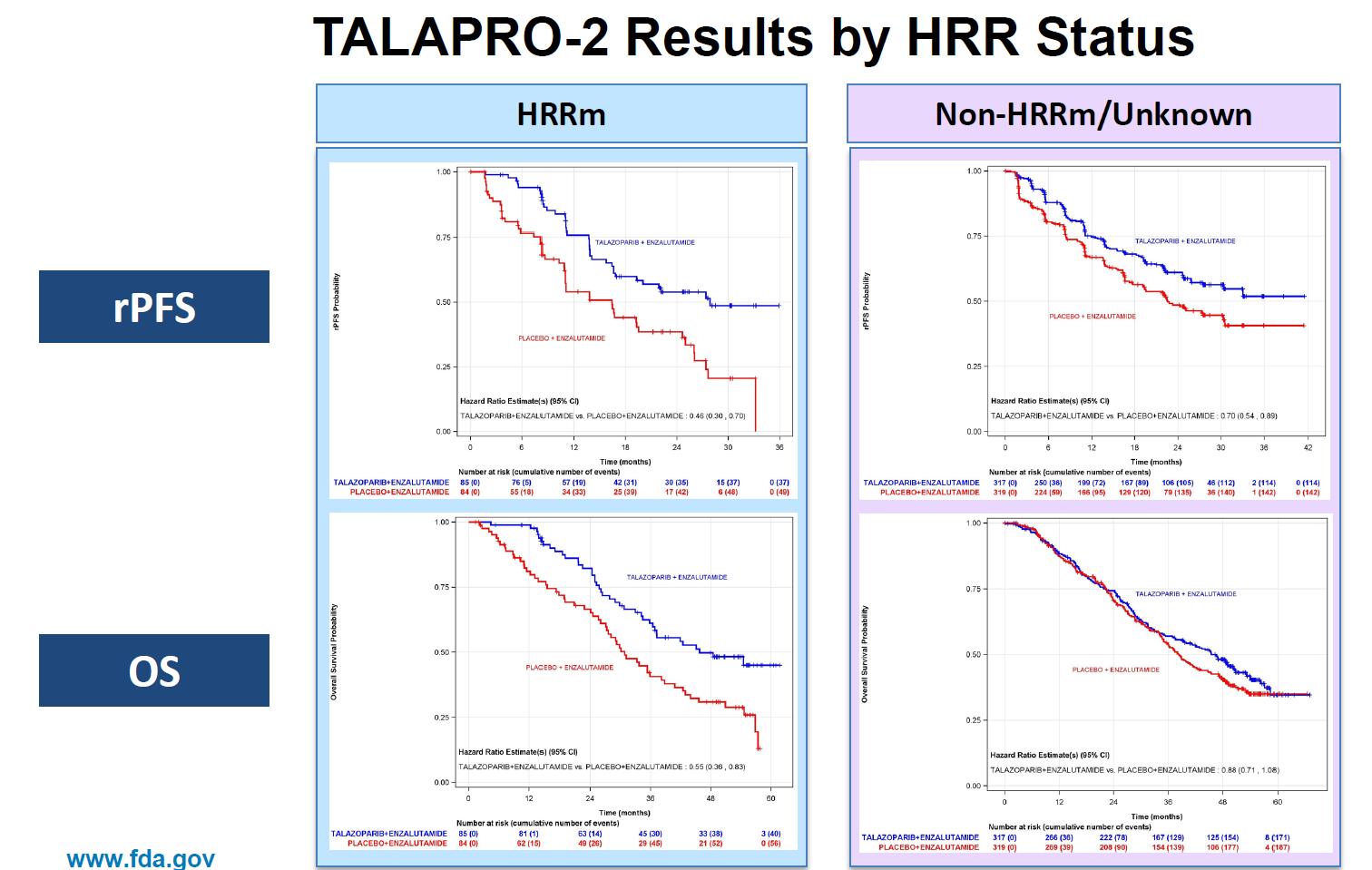
It seems a safe bet that Pfizer's PARP inhibitor latecomer Talzenna won't see its US label in first-line prostate cancer expanded beyond patients whose cancers harbour HRR mutations. On Wednesday a US advisory committee voted unanimously, 8-0, that the Talapro-2 study doesn't support approval in all-comers. Pfizer argues that Talapro-2 shows Talzenna plus Xtandi to confer a significant OS benefit over Xtandi alone both in all-comers and in HRR-mutant disease, but data at ASCO-GU confirmed that reduction in risk of death in the former (20%) was less than in the latter group (38%). The FDA's Jaleh Fallah told the adcom that the OS benefit in all-comers was "clearly attributed" to patients with HRR-mutant disease, echoing concerns of one of the investigators at ASCO-GU who stated that in patients with no detectable HRR alterations there was "no significant OS benefit". One curiosity is that Talzenna already has an all-comers EU label in first-line prostate cancer, following a 2023 CHMP recommendation, meaning that the FDA has taken a tougher stance. In February the FDA accepted Pfizer's Talzenna all-comers filing with priority review, and while no PDUFA date has been disclosed the timeline suggests that a decision is due in August.
1115













