
Lunsumio steps into Columvi territory
Sunmo succeeds in a setting very similar to Starglo's.
Sunmo succeeds in a setting very similar to Starglo's.

Roche had once positioned its two anti-CD20 T-cell engagers, Columvi and Lunsumio, for separate lymphoma settings, but the lines have recently become blurred. Now, with Friday's win for Lunsumio in the phase 3 Sunmo study, the drugs look set to compete for a share of the same pie.
That's because Sunmo concerns second-line diffuse large B-cell lymphoma patients ineligible for transplant – effectively the same setting for which Roche is seeking Columvi approval, backed by the Starglo trial. True, the two regimens differ, but with Roche confirming that Sunmo will be used to back a regulatory filing the company has a delicate balancing act on its hands.
Lunsumio has accelerated approval in relapsed follicular lymphoma, but the Sunmo trial concerns the more aggressive DLBCL indication for which Columvi has a third-line label, also on an accelerated basis. Having previously toplined Sunmo as positive, Roche on Friday revealed the data in full at a late-breaker during the International Conference on Malignant Lymphoma.
Sunmo combined Lunsumio with Polivy, and compared this against Rituxan, gemcitabine and oxaliplatin (R-Gemox). The key primary endpoint of progression-free survival showed a striking 59% reduction in risk of progression or death (p<0.0001), with median PFS of 11.5 months versus 3.8 months. On the other co-primary, ORR, Lunsumio won out by 70% to 40%.
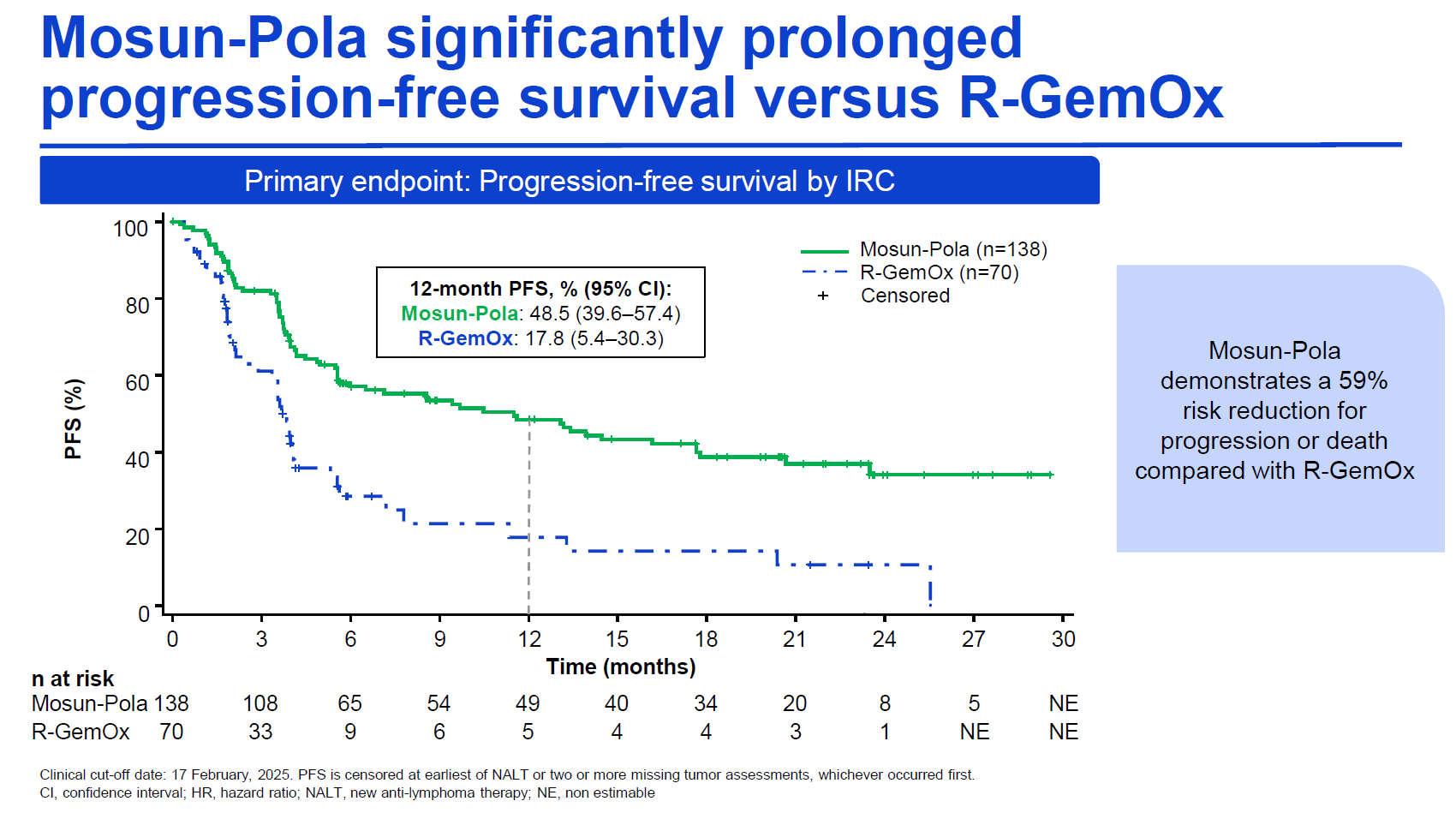
Source: Dr Jason Westin & ICML.
Importantly, overall survival also looked impressive, though at present the data are immature and haven't hit statistical significance. Median OS at this point was 18.7 months for Lunsumio plus Polivy, versus 13.6 months for R-Gemox. The Sunmo numbers broadly back uncontrolled phase 1/2 data, where Lunsumio plus Polivy scored mPFS of 11.4 months, and mOS of 23.3 months.
Roche argues that Sunmo is especially important because it could offer patients a chemo-free regimen. This differs from the second-line approval being sought for Columvi based on the Starglo trial, which also used R-Gemox as comparator, but whose experimental arm combined Columvi with Gemox.
Whether this approval comes is up in the air, after Starglo last month received a negative vote at an advisory committee meeting, which deemed that its population (over half of patients were Asian, and only 9% were from North America) wasn't applicable to the US.
Same again?
One highly relevant question is whether the company risks running into a similar problem with Lunsumio in Sunmo. According to the forest plot revealed at the ICML late-breaker, only 10% of Sunmo's 208 patients were from North America, with East Asia accounting for 38%, and Latin America 41%.
This will become something the FDA will have to deal with once a regulatory filing is submitted. The agency is currently grappling with the adcom vote on Starglo, which also serves as a possible confirmatory trial for Columvi monotherapy's late-line DLBCL use; Roche's Starglo-based filing faces a 20 July PDUFA date.
DLBCL is also still in play for Columvi in the first line, where the phase 3 Skyglo study combines it with Polivy. However, this isn't expected to read out until 2027, and the general picture emerging seems to be that Columvi's progress in DLBCL has been stymied.
In the US there's a two-way battle in DLBCL between Columvi and AbbVie/Genmab's rival anti-CD20 T-cell engager Epkinly – a battle Regeneron's odronextamab recently dropped out of, having been focused instead on follicular lymphoma. Epkinly is also approved in Lunsumio's setting of follicular lymphoma.
Roche is fortunate in having Lunsumio on hand to pick up any slack in DLBCL, but in terms of the addressable market the risk of seeing Columvi sales being cannibalised is real.
Selected settings for Roche’s anti-CD20 T-cell engagers
| Follicular lymphoma | 3L+ DLBCL | 2L+ DLBCL (transplant ineligible) | 1L DLBCL | |
|---|---|---|---|---|
| Columvi | NA | Ph1/2 NP3017 | Ph3 Starglo | Ph3 Skyglo |
| Monotherapy | Gemox combo, vs R-Gemox | Polivy + R-CHP combo, vs Polivy + R-CHP | ||
| US accelerated approval | Awaiting US approval (-ve adcom vote) | Data in 2027 | ||
| Lunsumio | Ph1/2 GO29781 | NA | Ph3 Sunmo | NA |
| Monotherapy | Polivy combo, vs R-Gemox | |||
| US accelerated approval | +ve for PFS & ORR |
Source: OncologyPipeline.
1105













