
How MiNK stole the NK T show
A single but durable stable disease sends the microcap's stock up 730%.
A single but durable stable disease sends the microcap's stock up 730%.

An intriguing case report of long-term disease stabilisation in a patient treated with AGENT-797 sent the shares of the Agenus spinout MiNK Therapeutics up 730% on Friday. True, MiNK remains a biotech minnow, capitalised at $255m even after the surge and sitting 45% below its 2021 IPO, but the report is a rare success for an NK T-cell therapy.
NK T cells make up a tiny part of the human immune system, and though they are thought to have some unique properties an earlier attempt to structure a biotech company around them ended in bankruptcy. MiNK floated on the promise of AGENT-797, an expanded but unmodified invariant NK T-cell therapy, but an earlier signal of efficacy met with zero investor reaction.
That concerned phase 1 data presented at AACR in 2023, and updated at SITC in 2023 and 2024, in 34 solid tumour patients given AGENT-797 with or without Opdivo. This included a third-line gastric cancer patient, who despite relapsing on Keytruda, and then failing to respond to Opdivo plus chemo, developed a partial response to AGENT-797 plus Opdivo.
Two-year stable disease
Now comes the case report from another patient from the same study, this one with metastatic testicular cancer and relapse on chemo, then relapse after a response to Keytruda plus chemo, another relapse on chemo with autologous stem cell transplantation, no response to Opdivo plus Yervoy, and no response to an undisclosed anti-TIGIT therapy.
The report, published in the Nature journal Oncogene, reveals that despite such heavy pretreatment a single infusion of AGENT-797 plus Opdivo put this patient into "complete remission clinically, radiologically and biochemically".
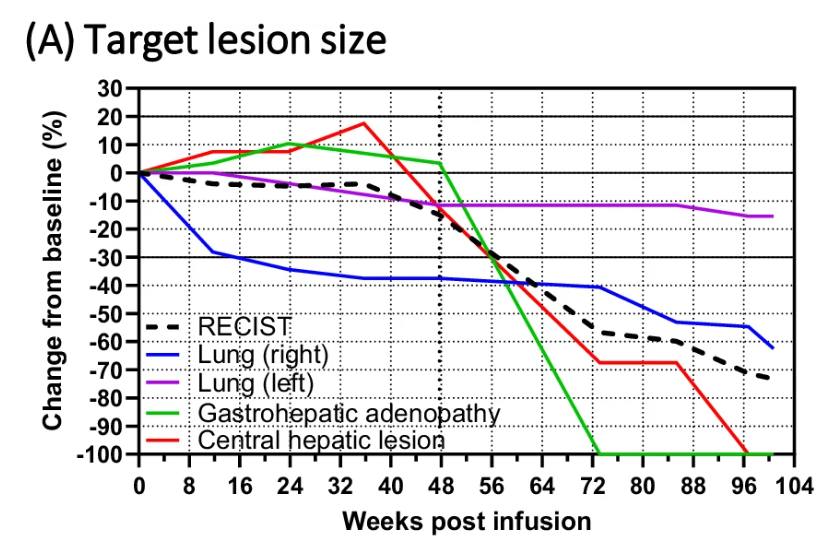
That said, target lesion reduction didn't meet the criteria for a formal complete response by Recist, and a lung metastasis appears to have shown no shrinkage, according to a graphic depicting target lesion size. But the patient remains with no tumour progression, and normalisation of a biochemical marker over two years later.
At the time of the SITC poster, which related to a 2 October 2023 cutoff, this testicular cancer patient was scored as a "stable disease"; an update at last year's SITC, which didn't specify a cutoff date, also reported stable disease as best response among testicular cancer subjects.
Another caveat is that the disease stabilisation might not have been due to the AGENT-797 part of the combo. The authors scotch this, stating that while Opdivo "may have contributed to a remarkable outcome this is unlikely due to progression on ... previous anti-PD-1 therapy". The claim is that NK T cells might hold the key to the holy grail of making tumours more immunogenic.
Change in tumour size in testicular cancer patient given AGENT-797 + Opdivo
Invariant NK T cells are a distinct T-cell population, said to combine durable memory responses with features of NK cells. They require no HLA matching, and in MiNK's study are administered without the need for lymphodepletion.
Their dual role, activating NK cells as well as CD8+ (cytotoxic) T cells, has been said to allow them to target both cancer and immunosuppressive components of the tumour microenvironment such as tumour-associated macrophages and myeloid-derived suppressor cells. This seems central to turning "cold" tumours "hot".
However, one notable company launched with a mission to exploit NK T cells didn't end well. That was the UK's Cell Medica, which had a partnership with Baylor College before being renamed Kuur Therapeutics; Kuur was then acquired by Athenex, which filed for Chapter 11 bankruptcy in May 2023.
Cell Medica/Kuur/Athenex's work centred on NK T cells modified to express a Car, and while MiNK's pipeline also includes a Car-NK T-cell therapy the focus remains on unmodified cells. It will be interesting to see if last week's publication allows MiNK to tap the markets for more cash.
2450













