World Lung 2025 – crossing curves in lung cancer
Tolerability could decide first-line therapy in EGFRm disease.
Tolerability could decide first-line therapy in EGFRm disease.

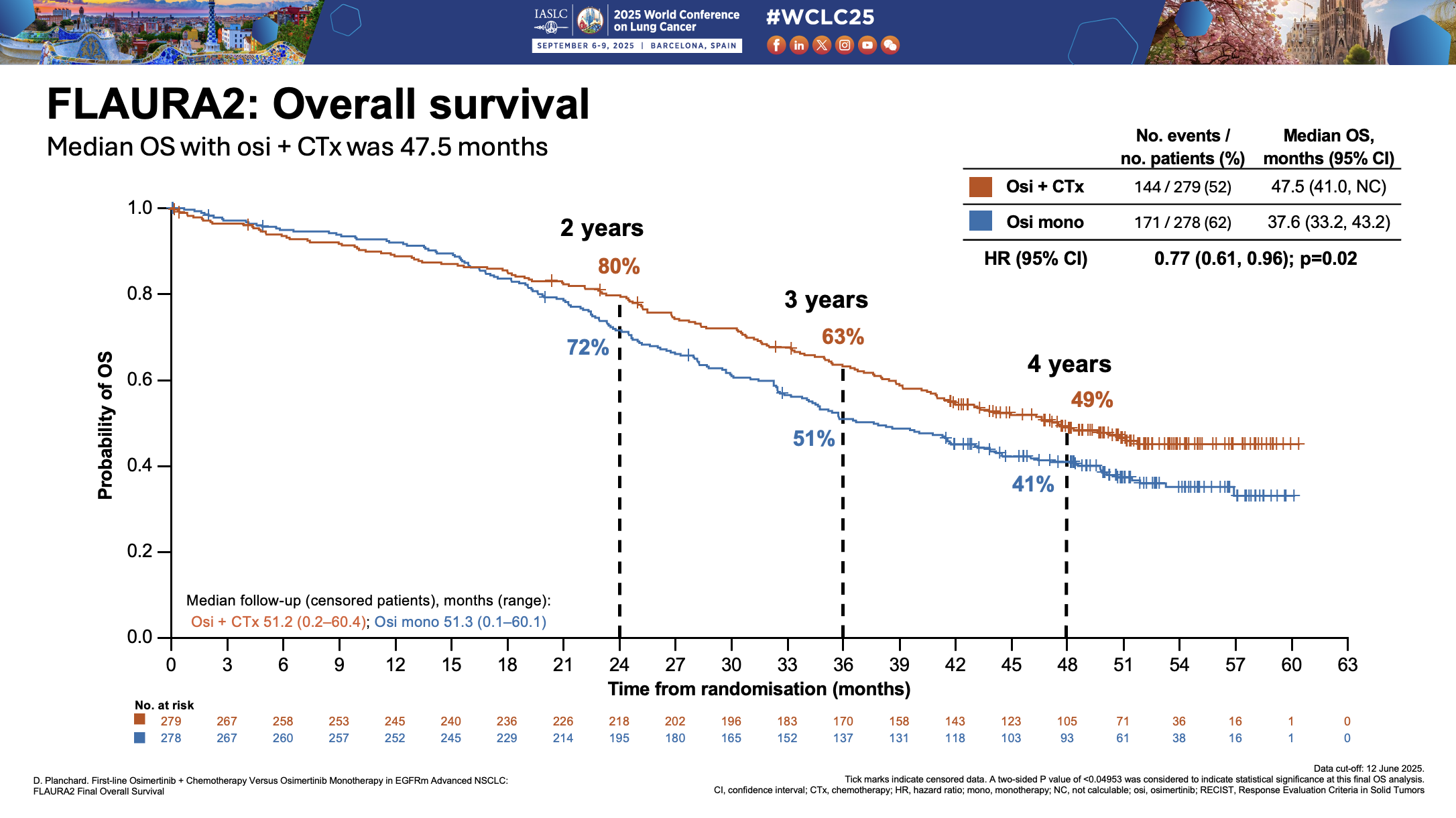
With final median overall survival data from AstraZeneca's Flaura2 study presented on Sunday at the World Congress on Lung Cancer, oncologists now have two pivotal trials with remarkably similar overall survival outcomes to consider.
Astra had already revealed that its trial, which evaluated Tagrisso’s combination with chemotherapy versus Tagrisso monotherapy in first-line EGFR-mutated NSCLC, was statistically significant and clinical meaningful for overall survival. Now full details have been revealed at a World Lung presidential symposium, showing similarity to a key rival, Johnson & Johnson's Mariposa study of Rybrevant plus Lazcluze.
Both J&J's Rybrevant/Lazcluze doublet and Astra's chemo combo are already approved in this setting, but only the former has OS data on its US label. While Mariposa delivered an impressive OS benefit with Rybrevant/Lazcluze versus Tagrisso monotherapy, one issue was that its survival curves crossed over, suggesting an initial detriment for Rybrevant/Lazcluze patients.
Now Astra has disclosed the full details from Flaura2, including its OS curves. At 57% maturity, the Tagrisso/chemo combo achieved a median overall survival of 47.5 months versus 37.6 months for Tagrisso monotherapy, with a three-year survival rate of 63% vs 51%.
Mariposa curves mirrored?
In Mariposa, Rybrevant plus Lazcluze hasn't reached median overall survival, though three-year survival was 60% versus 51%. However, just like Mariposa, Flaura2's survival curves cross over, at a broadly similar point to Mariposa's, to the extent that the curves of the two trials appear to mirror one another.
Asked ahead of the conference about this crossover in the Mariposa trial, Mark Wildgust, vice-president of global medical affairs for J&J oncology, told ApexOnco: “That’s just noise in the system – it’s just a few patients who were a little bit sicker in one arm versus the other arm.”
Ultimately, the strikingly similar patterns in the two trials suggest that the choice of first-line regimen for EGFR-mutated patients might rest less on efficacy and more on tolerability.
Chemotherapy has long been linked with a wide range of adverse events, though Rybrevant also carries its own risks, including severe rash and venous thromboembolic events – issues that J&J is trying to address with prophylactic therapies, in the Cocoon and Copernicus studies.
J&J is also claiming a lower emergence of EGFR and cMet resistance mutations with Rybrevant plus Lazcluze. At World Lung on Saturday that group presented a new analysis of Mariposa, showing that 3% and 1% of patients receiving Rybrevant/Lazcluze developed cMet amplifications and secondary EGFR mutations respectively, versus 13% and 8% with Tagrisso monotherapy.
Wildgust contended that this could be another important consideration for doctors looking to give patients their "best first chance", without potentially affecting subsequent lines of therapy.
Still, for clinicians various factors will come into play, with patients’ morbidities, age and preferences likely to play decisive roles in selecting the most suitable regimen.
Mariposa vs Flaura2 in first-line EGFRm NSCLC
| Mariposa | Flaura2 | |||
|---|---|---|---|---|
| Regimen | Rybrevant + Lazcluze | Tagrisso | Tagrisso + chemo | Tagrisso |
| mPFS (months) | 23.7 | 16.6 | 25.5 | 16.7 |
| Stats | HR=0.70; p=0.002 | HR=0.62; p<0.001 | ||
| mOS (months) | Not reached | 36.7 | 47.5 | 37.6 |
| Stats | HR=0.75; p<0.005 | HR=0.77; p<0.002 | ||
Source: ELCC2025, WCLC 2025 & OncologyPipeline.
This story has been updated.
2193