
ASCO 2025 – Enhertu mounts its first-line charge
Enhertu plus Perjeta shows a convincing progression-free survival benefit over a Perjeta triplet.
Enhertu plus Perjeta shows a convincing progression-free survival benefit over a Perjeta triplet.

AstraZeneca and Daiichi Sankyo’s HER2-targeting ADC Enhertu, which made $3.8bn in 2024, could soon be heading for first-line breast cancer, following positive data presented at ASCO on Monday from the Destiny-Breast09 trial.
The study, in HER2-positive disease, found a 44% reduction in the risk of progression or death with Enhertu plus Roche’s Perjeta, versus Perjeta plus Herceptin and chemo. Furthermore, the control arm outperformed historical data with Perjeta/Herceptin/chemo, making Enhertu’s win seem even more convincing.
Presenting the data at an ASCO press conference, Dr Sara Tolaney of the Dana-Farber Cancer Institute said that Enhertu plus Perjeta could become a first-line standard of care in this population.
At a media event ahead of ASCO, Astra’s head of oncology R&D, Susan Galbraith, declined to give a timeline for regulatory filings based on Destiny-Breast09, but said discussions with regulators were ongoing, and that an announcement would be made on acceptance.
In Destiny-Breast09 the Enhertu/Perjeta doublet produced a median PFS of 40.7 months, versus 26.9 months with Perjeta/Herceptin/chemo (either paclitaxel or docetaxel). This hit statistical significance with a p value under 0.00001.
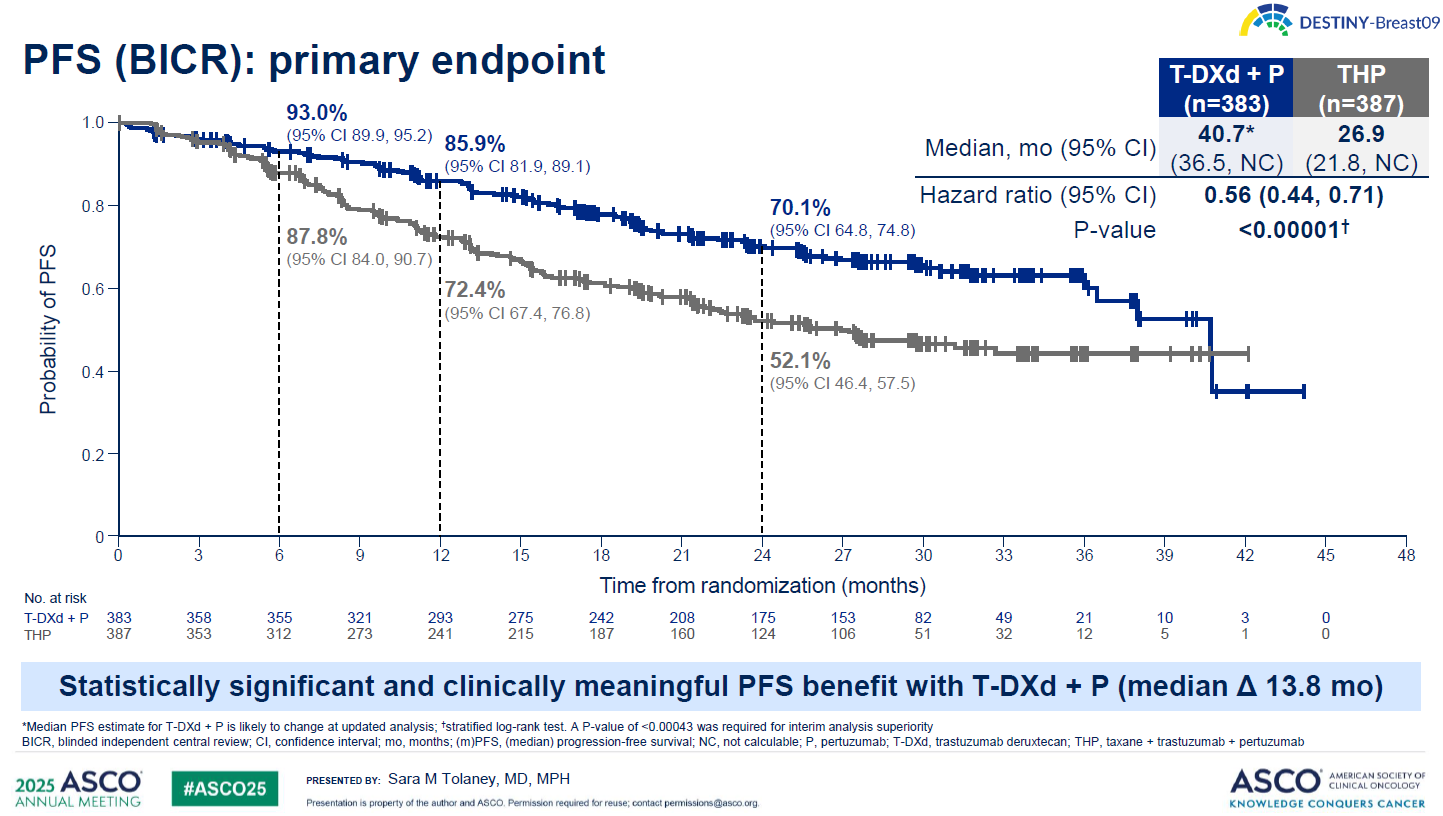
Meanwhile, Perjeta’s label cites mPFS of 18.5 months with a Perjeta/Herceptin/docetaxel combo in the Cleopatra trial, in first-line HER2-positive breast cancer.
Overall survival in Destiny-Breast09 was immature, with only 16% having died, but Astra cited an “early survival trend” for the Enhertu combo, with a hazard ratio of 0.84.
The rates of grade 3 or higher possibly treatment-related adverse events were similar between the two arms, but there were more deaths in the Enhertu cohort, with five deemed possibly treatment related, versus one in the control arm.
Cross-trial comparison in first-line HER2-positive metastatic breast cancer
Destiny-Breast09 | Cleopatra | |||
|---|---|---|---|---|
| Enhertu + Perjeta | Perjeta + Herceptin + chemo | Perjeta + Herceptin + chemo | Herceptin + chemo | |
| mPFS | 40.7 months | 26.9 months | 18.5 months | 12.4 months |
| Stats | HR=0.56; p<0.00001 | HR=0.62, p<0.0001 | ||
Source: ASCO 2025, Perjeta label.
Discussing the results at the press conference, Dr Rebecca Dent of the National Cancer Centre Singapore mentioned the toxicities of the regimen – in terms of quality of life, but also the cost toxicity. Despite this, she deemed the results impressive.
The trial also tests Enhertu monotherapy versus the triplet control, but no data were disclosed here.
Astra estimated that, in the G7 countries, around 23,000 patients have first-line HER2-positive metastatic breast cancer.
The company is looking even more broadly, though, with the neoadjuvant and adjuvant Destiny-Breast11 and Destiny-Breast05 trials. The former was toplined positive in May, while Destiny-Breast05 is due to yield data in the second half.
3254













