
Tango waltzes towards phase 3
The company plans a pivotal pancreatic cancer trial after seeing two responses in eight patients.
The company plans a pivotal pancreatic cancer trial after seeing two responses in eight patients.

Tango Therapeutics believes that it’s shown enough to move its next-generation PRMT5 inihibitor vopimetostat into a pivotal pancreatic cancer trial, but investors appeared less enthused. The group's stock sank 13% on Thursday on the group's latest update, although the announcement of a $210m equity offering also can't have helped.
The company, which also raised an additional $15m in a private placement, reported data on Thursday from a phase 1/2 trial of vopimetostat. This amounted to a 27% ORR across 94 evaluable patients with various MTAP-deleted cancers; it included unconfirmed responses, and looked in line with a 23% ORR with Bristol Myers Squibb’s BMS-986504 across various solid tumours.
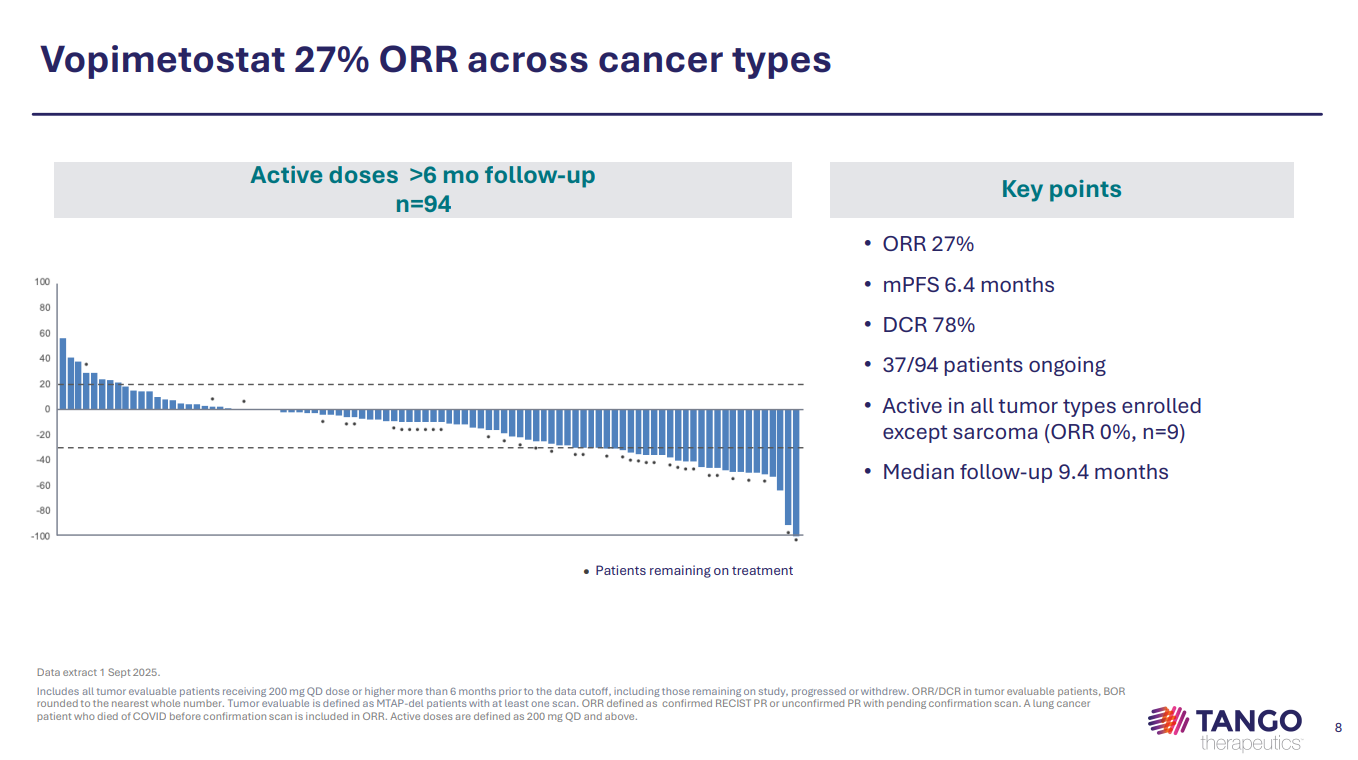
Tango zoomed in on second-line pancreatic cancer, where it claimed a 25% ORR. The group didn’t spell out whether these were confirmed responses, or provide a waterfall plot for pancreatic cancer specifically.
A closer look at the 25% ORR reveals that this amounts to two responses in eight subjects, out of a cohort of 29 second-line patients treated with "active doses" of vopimetostat.
Across all pancreatic cancer patients, ORR was a less impressive 15%. The denominator here appears to include 29 evaluable third-line plus patients, out of 34 treated with the "active doses".
In pancreatic cancer another PRMT5 rival, Amgen’s AMG 193, has produced a confirmed ORR of 9% at active doses among 23 patients – but this rose to 22% if unconfirmed responses were also included.
Second-line MTAP-deleted pancreatic cancer will be the setting for Tango’s first pivotal study of vopimetostat, a non-brain-penetrant PRMT5 inhibitor formerly known as TNG462. The new trial, slated to start in 2026, will enrol around 300 patients and compare vopimetostat 250mg daily versus investigator’s choice of chemo.
According to Tango, chemo has historically produced an ORR of around 10% in second-line pancreatic cancer, so this is the bar vopimetostat will need to beat.
Lung and combos coming
Meanwhile, the phase 1/2 study is additionally testing vopimetostat in second-line NSCLC, with an update expected next year.
PRMT5 inhibition has largely been seen as disappointing so far, with Tango last year discontinuing its then lead project, the brain-penetrant PRMT5 inhibitor TNG908, after this disappointed in glioblastoma.
However, Bristol has already moved BMS-986504, which it gained via the acquisition of Mirati, into a phase 2/3 trial. And early results could also come this year with AstraZeneca’s AZD3470.
2013













