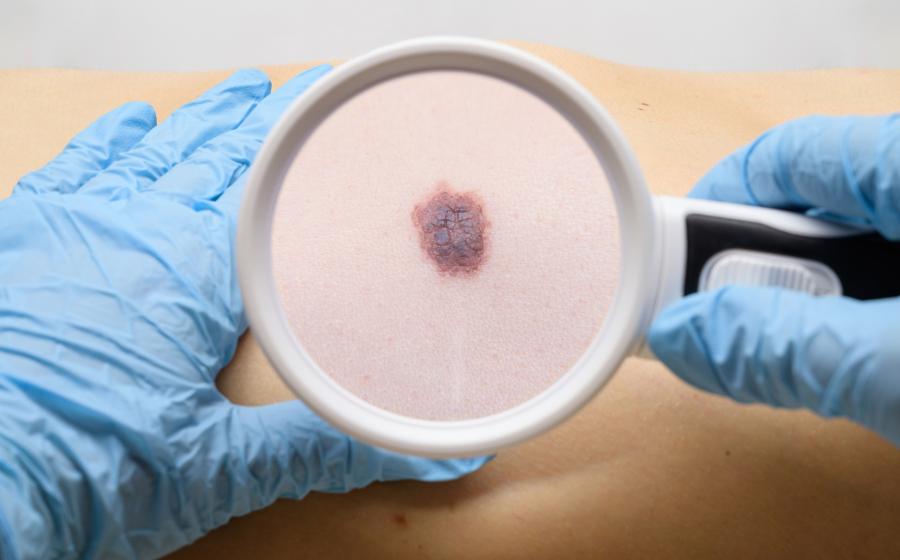
Moderna takes on IO Biotech
The group is expanding its mRNA-4359 trial to include first-line melanoma.
More problems for BioNTech & Roche’s neoantigen shot
Autogene cevumeran goes on hold in adjuvant bladder cancer.
TuHura makes its bid for Merkel accelerated approval
The company reckons its immunotherapy could improve on Keytruda.
Scancell’s dilemma delayed
The group touts a 72% ORR with SCIB1, but full Scope data have been pushed back into 2025.