
World Lung 2025 – Ideaya challenges Zai Lab
Early data with IDE849 look better those with Zai’s zocilurtatug pelitecan.
Early data with IDE849 look better those with Zai’s zocilurtatug pelitecan.

Zai Lab, leading the increasingly crowded anti-DLL3 ADC field, now has a new challenger in Ideaya. A late-breaking presentation at the World Conference on Lung Cancer on Sunday shows Ideaya’s IDE849, licensed from Jiangsu HengRui, producing early data in relapsed small-cell lung cancer that look better than with results with Zai’s zocilurtatug pelitecan.
There are the usual caveats about cross-trial comparisons, and both assets have much to prove as they chase down Imdelltra, Amgen’s approved DLL3-targeting T-cell engager. But there now look to be two legitimate ADC contenders – and Roche is also jostling for position here, via a deal with Innovent over IBI3009 (now known as RG6810).
After being up 15% premarket on Monday, Ideaya's ended the day down 14%, although the share price could also have been impacted by phase 2 data with the group's lead project, daravosertib, in neoadjuvant uveal melanoma. The company, which had an R&D day on Monday, also released phase 1/2 data with its MAT2A inhibitor IDE397 plus Trodelvy in MTAP-deletion urothelial cancer.
Safety first
There are a few reasons to be caution about IDE849, previously known as SHR-4849, which Ideaya licensed outside China for $75m up front. First, the HengRui-sponsored phase 1 trial presented at World Lung only enrolled Chinese patients, so it can be questioned whether the findings are applicable to a global population.
Second, the incidence of grade 3 or higher treatment-related adverse events looked relatively high, at 48%, the most common being reductions in white blood cells. There was also a dose-limiting toxicity of grade 4 decreased platelet count at 4.2mg/kg, the highest of five doses tested.
On the positive side, there were no treatment-related deaths.
The study included a handful of patients with neuroendocrine carcinoma, but Dr Linlin Wang of Shandong Cancer Hospital, presenting the results, focused on 72 evaluable SCLC patients who received doses of 0.8-4.2mg/kg (although one only patient got the apparently subtherapeutic 0.8mg/kg dose).
At a cutoff date of 20 June there were 52 responses, giving an impressive ORR of 73%.
However, only 34 of these responses were confirmed, and this gave an ORR of 47%. With 10 responses still pending confirmation it’s possible that the confirmed ORR could tick up.
Ideaya focused on a 35-patient strong second-line cohort, which saw a confirmed ORR of 60% at 2.4mg/kg and above.
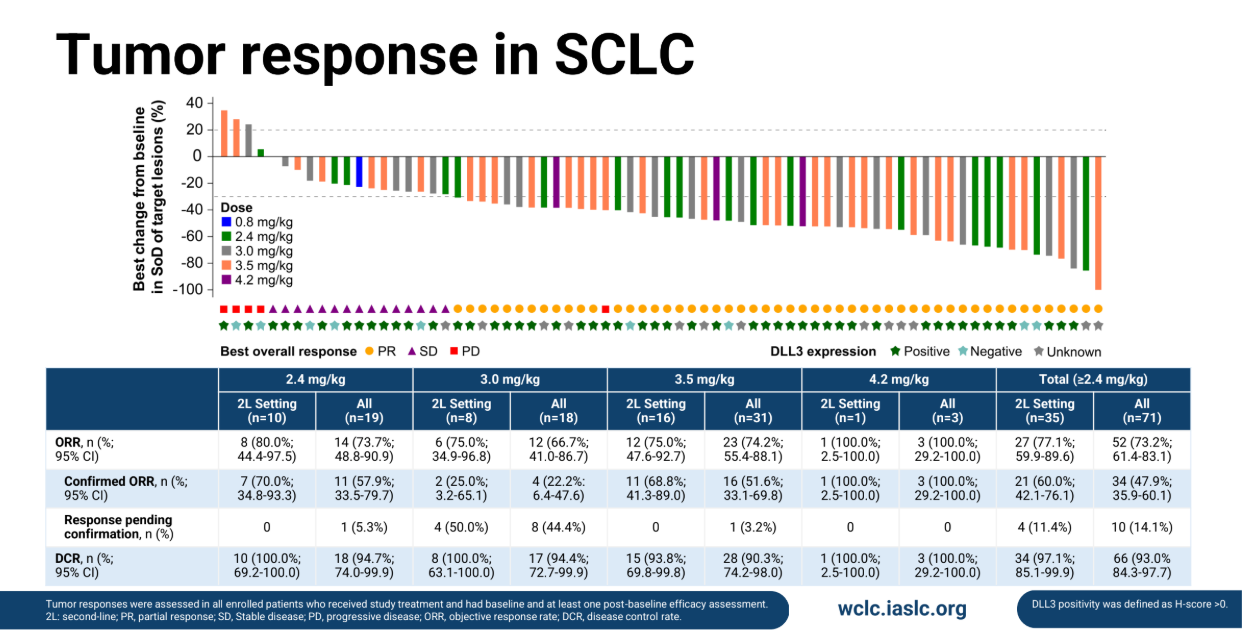
Meanwhile, median progression-free survival was 6.7 months across therapy lines, at ≥2.4mg/kg. On the face of it, these data appear better than those seen with Zai’s zoci-P, most recently at ASCO, though they do include the 4.2mg dose, which appears unrealistic given its toxicity.
In Zai's global phase 1 study there was a 51% ORR across doses – but this dropped to 37% if only confirmed responses were counted. Meanwhile, there was a 57% confirmed ORR in second-line patients receiving a 1.6mg/kg dose, but this number came from a small sample of 14 subjects.
Cross-trial comparison of DLL3-targeting ADCs in relapsed SCLC
| IDE849 (SHR-4849) | zocilurtatug pelitecan (ZL-1310) | |
|---|---|---|
| Company | Ideaya/ Jiangsu HengRui | Zai Lab |
| Description | ADC | ADC |
| Trial | China ph1 | Global ph1 |
| Presentation venue | World Lung 2025 | ASCO 2025 |
| ORR* | 72% (52/72)** | 51% (38/74)^ |
| Confirmed ORR | 47% (34/72)** | 37% (27/74)^ |
| Gr≥3 TRAEs | 48% | 23% |
| TRAE leading to discontinuation | 2% | 0% |
Notes: both trials uncontrolled; *confirmed + unconfirmed responses; **across 0.8-4.2mg/kg; ^across 0.8-2.8mg/kg. Source: OncologyPipeline, ASCO & WCLC.
Zai has previously said that zoci-P, previously known as ZL-1310 and which it licensed from MediLink, could be safer and at least as efficacious, if not better than Imdelltra – and Ideaya likely hopes to show this with IDE849, too.
Imdelltra has produced a 35-40% ORR, and median PFS of 4.2 months, and its label carries warnings of cytopenias, infections and hepatotoxicity. Furthermore, patients must be closely monitored because of the risk of cytokine release syndrome.
Still, Amgen’s confirmatory Dellphi-304 trial shifted from monitoring patients for 48 hours in hospital, to outpatient monitoring over six to eight hours. And the ADCs don’t seem immune from cytopenias either.
There are also questions about whether Zai will be able to file for accelerated approval of zoci-P, as planned, with Imdelltra now seemingly headed for full approval following strong overall survival results from Dellphi-304.
Ideaya has said it's started a US phase 1 trial of IDE849 in SCLC, although this doesn't yet appear to have been listed on clinicaltrials.gov. With the ideal dose of the project still being nailed down, the group has a way to go – but for a relatively modest outlay it looks to have secured a decent DLL3 contender.
This story has been updated to include results from longer-term follow-up.
2235













