
Arcus reaches the end of the road for etrumadenant

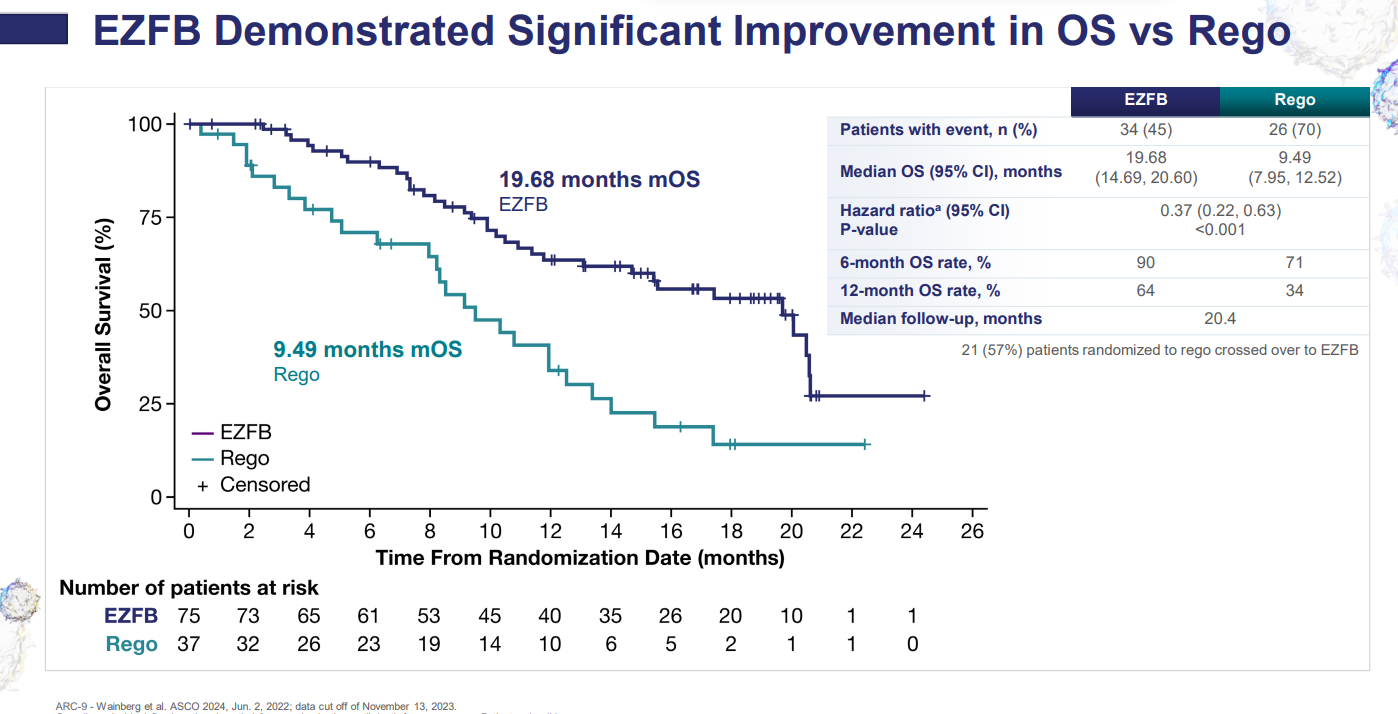
Arcus had once touted etrumadenant as a potentially best-in-class adenosine receptor antagonist, but now seems to have admitted defeat with the project. The company’s second-quarter earnings release noted a “pause” in development following a decision not to push into phase 3 in third-line colorectal cancer, and the exit of the group’s partner Gilead. Arcus had hoped for a path forward based on the phase 1/2 Arc-9 trial, which at ASCO 2024 showed a 73% reduction in the risk of death with etrumadenant plus zimberelimab (Arcus’s anti-PD-1) plus chemo and Avastin, versus Stivarga. Arcus met the FDA in March, but it appears the agency requested a phase 3 trial, which was enough to prompt Gilead to hand back rights. The big biotech also this year declined to exercise an option on Arcus’s HIF2α inhibitor casdatifan, although the groups are still collaborating on the aforementioned zimberelimab, the CD73 inhibitor quemliclustat, and the anti-TIGIT antibody domvanalimab. However, TIGIT hopes aren’t high following the failure of various similarly acting projects, while Gilead seems to have cooled on quemliclustat, with the phase 3 Prism-1 pancreatic cancer trial Arcus’s sole responsibility as per a January 2024 investment reshuffle.
Arc-9 data at ASCO 2024
2294













